e− configuration of .90 Th-
A
(n−2)f2,(n−1)d1ns1
B
(n−2)f1,(n−1)d1ns2
C
(n−2)f0,(n−1)d2ns2
D
(n−2)f2,(n−1)d0ns2
Video Solution
Text Solution
The correct Answer is:C
|
Answer
Step by step video, text & image solution for e^(-) configuration of ._(90) Th- by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The number of αandβ-particles emitted during the transformation of .90Th232 to .82Pb208 are respectively
A4, 2B2, 2C8, 6D6, 4An isotope of .90Th231 can be converted into .90Th227 by the emission of
Aone α-particleBone β-particleCtwo α and one β-particleDone α and 2β- particlesA radioactive disintegration of 90Th232 yields 82Pb208 in the end . The number of α and β -particle emitted will be
A6α and 6βB5α and 5βC6α and 4βD4α and 6β
Electronic configuration of anatom
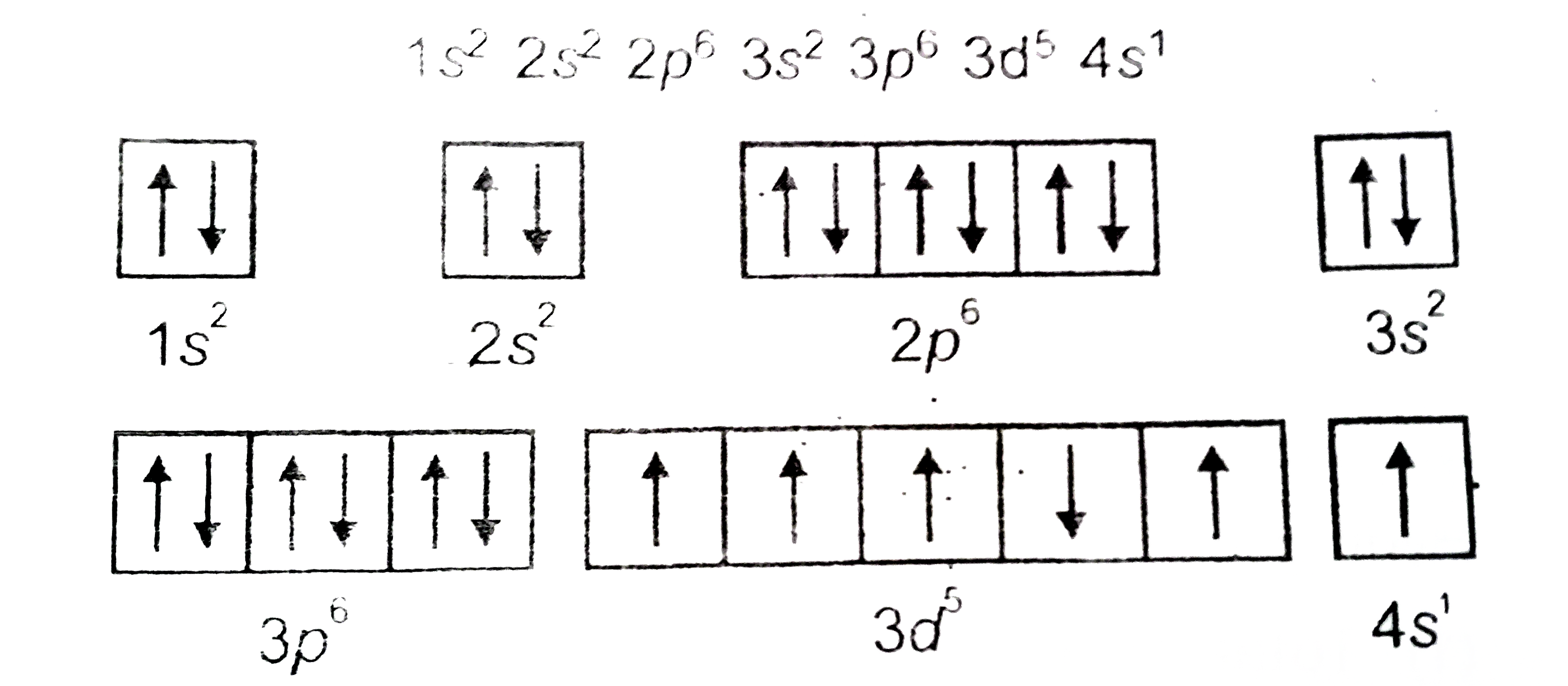
Choose the correct statement regarding this E.C.
View SolutionAssign E−Z configuration to each of the following:
a.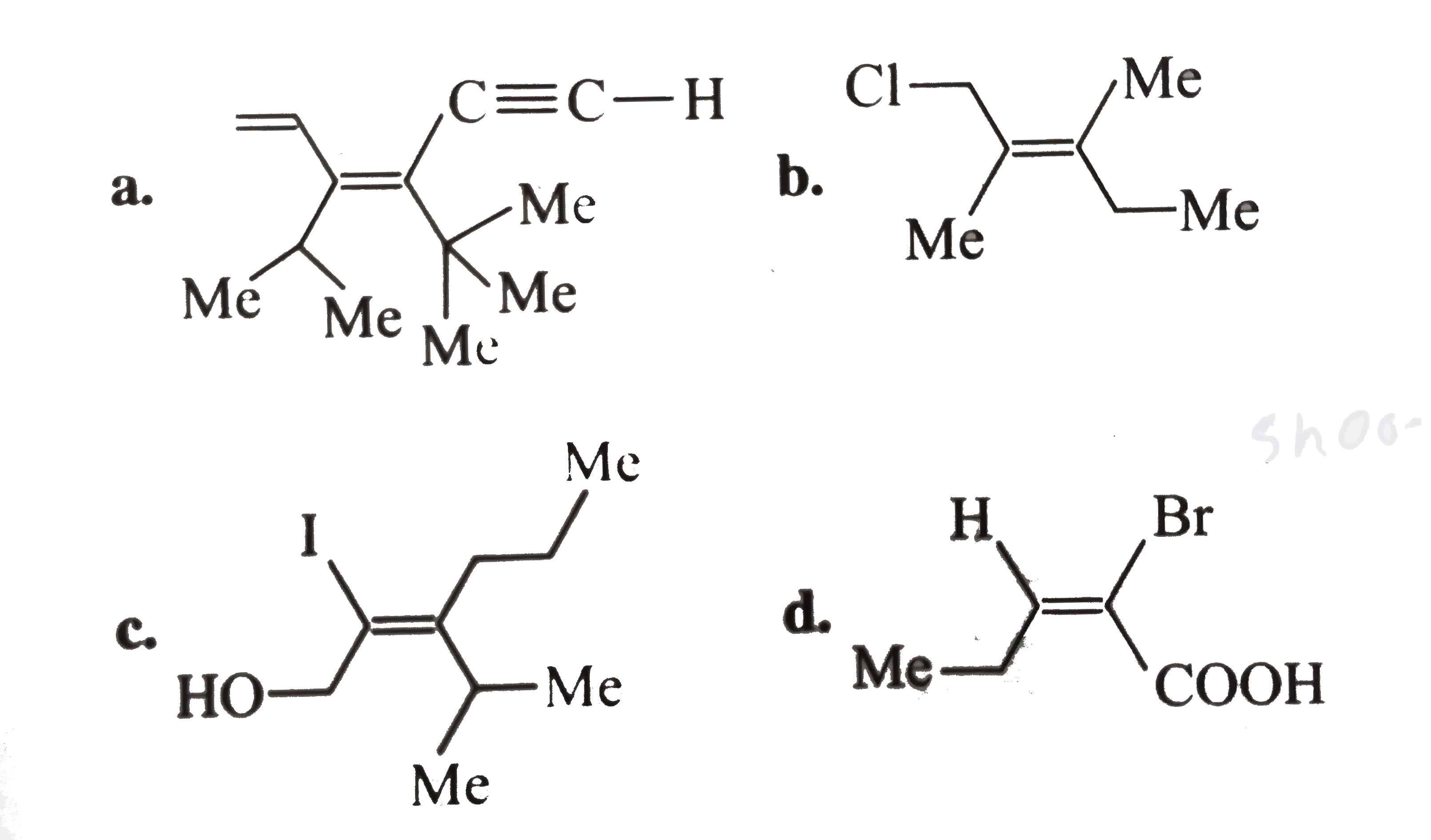
View SolutionHow may α− and β− particles will be emitted when .90Th232 changes into .82Pb208?
View Solution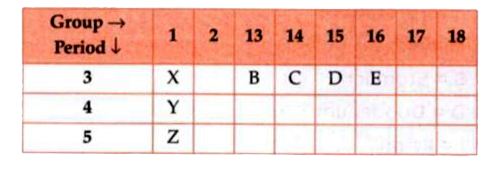
Write electronic configuration of element E.
View SolutionTh electroniv configuration of Mn can be written as
View Solution
