Cyclooctatetraene is not aromatic because
A
It has four carbn-carbon double bonds
B
It has not got (4n+2)π electrons (where n = 1,2,3, . . . . Ect) and is non planar
C
It is planer in structure
D
It decolouriese potassium permagnate solution
Video Solution
Text Solution
The correct Answer is:B
|
Answer
Step by step video, text & image solution for Cyclooctatetraene is not aromatic because by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Knowledge Check
An aromatic molecule will
Ahave 4nπ−electronsBhave (4n+2)π−electronsCbe planarDbe cyclicThe radical
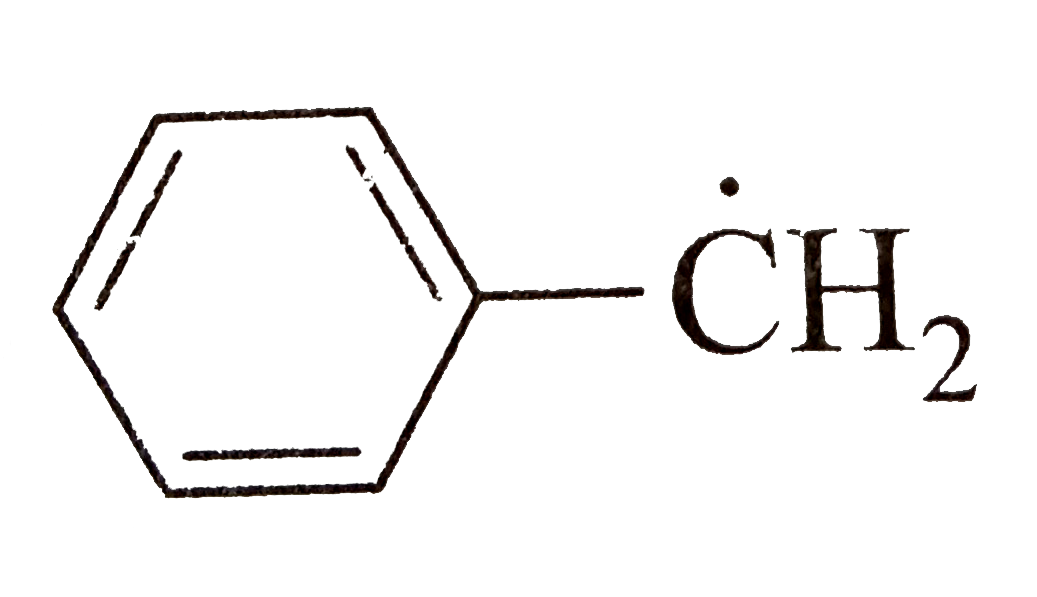 is aromatic because it hasA7 p-orbitals and 6 unpaired electronsB7 p-orbitals and 7 unpaired electronsC6 p-orbitals and 7 unpaired electronsD6 p-orbitals and 6 unpaired electrons
is aromatic because it hasA7 p-orbitals and 6 unpaired electronsB7 p-orbitals and 7 unpaired electronsC6 p-orbitals and 7 unpaired electronsD6 p-orbitals and 6 unpaired electronsThe radical,
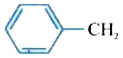 is aromatic because it has A7 p - orbitals and 6 unpaired electronsB7 p - orbitals and 7 unpaired electronsC6 p - orbitals and 7 unpaired electronsD6 p - orbitals and 6 unpaired electrons
is aromatic because it has A7 p - orbitals and 6 unpaired electronsB7 p - orbitals and 7 unpaired electronsC6 p - orbitals and 7 unpaired electronsD6 p - orbitals and 6 unpaired electrons


 is not aromatic ?
is not aromatic ?