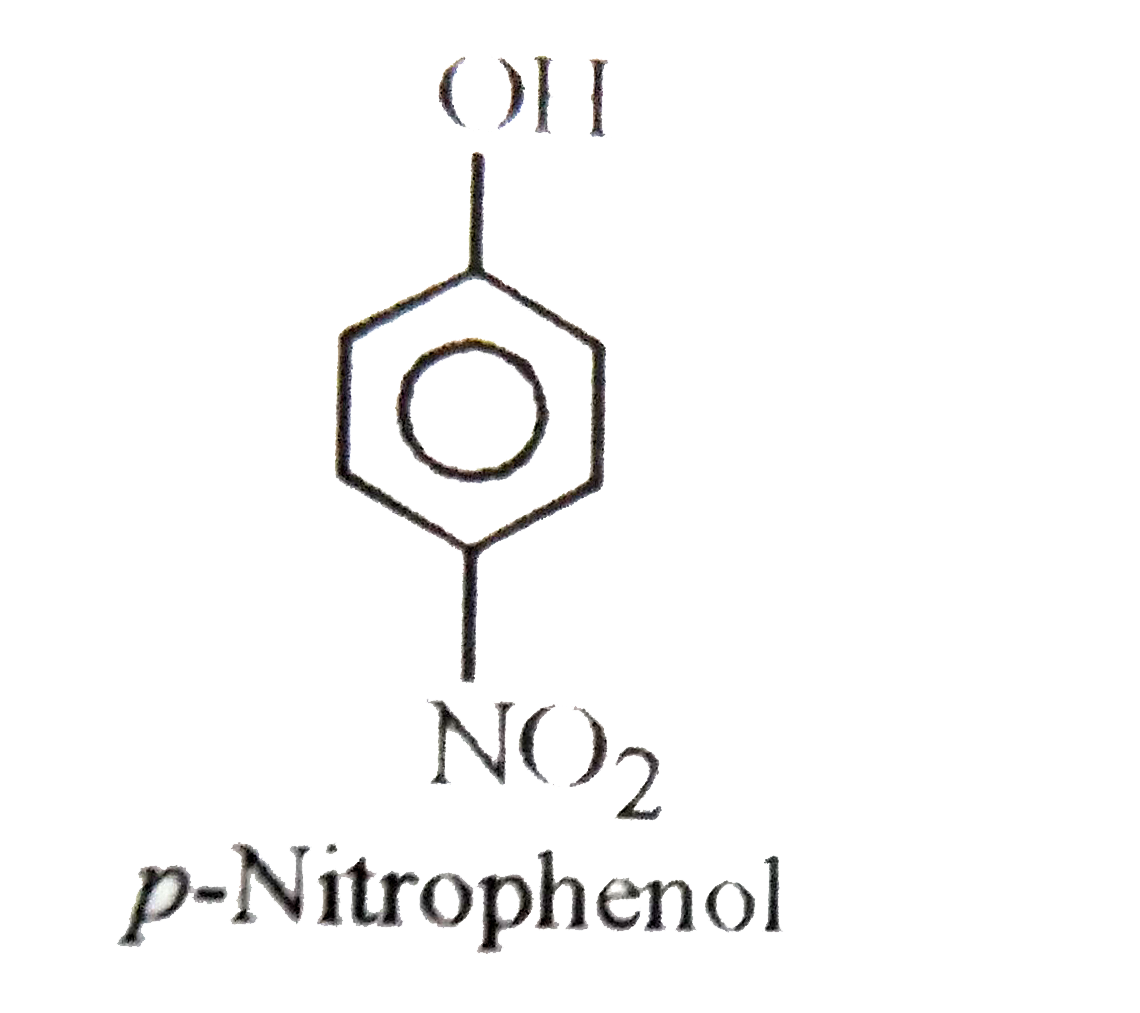
Which of the following is more stronger acid than Phenol ?
A
Ethanol
B
Phenylethanol
C
p-Nitrophenol
D
p-Cresol
Text Solution
The correct Answer is:C
|
Topper's Solved these Questions
- ALCOHOLS AND PHENOLSBook:DINESH PUBLICATIONChapter:ALCOHOLS AND PHENOLSExercise:RQ
Explore 225 Videos
- ALCOHOLS AND PHENOLSBook:DINESH PUBLICATIONChapter:ALCOHOLS AND PHENOLSExercise:Selected Straight Objective Type MCQs
Explore 55 Videos
- APPENDICESBook:DINESH PUBLICATIONChapter:APPENDICESExercise:Multiple choice
Explore 76 Videos
Similar Questions
Knowledge Check
Which of the following is stronger acid than methanol?
ACH3−C≡CHB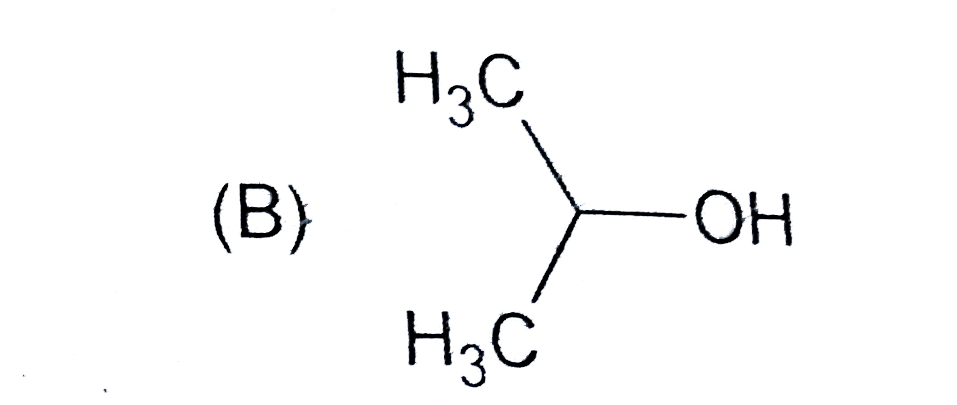 C
C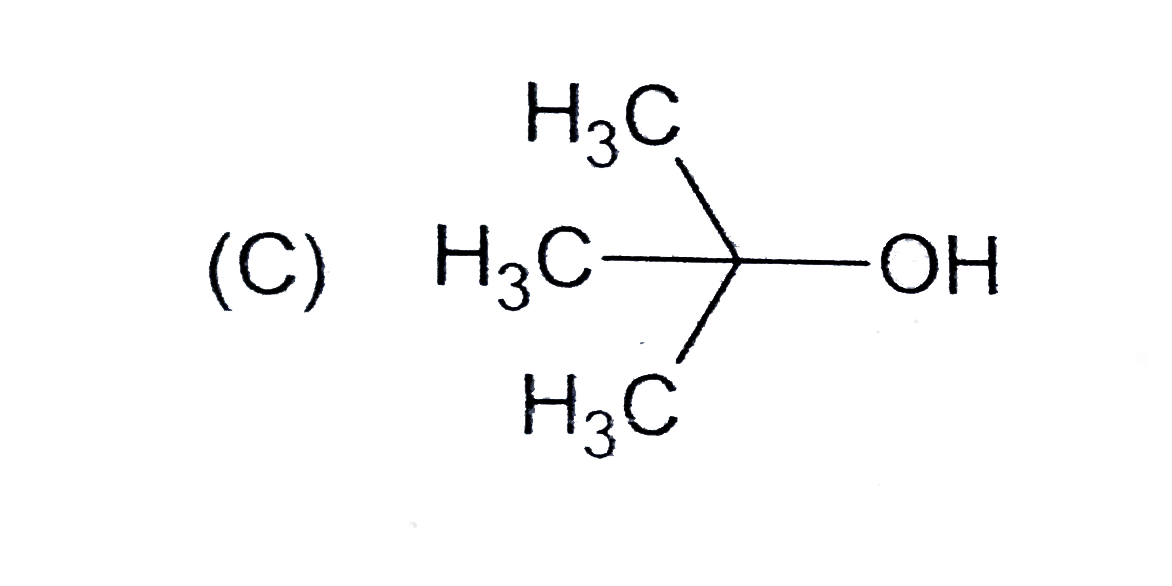 DH3C−O∣h+−H
DH3C−O∣h+−HWhich of the following is more acidic than phenol?
Ap-nitrophenolBEthanolCCresolDBenzyl alcoholp-Chlorophenol is stronger acid than phenol because
ACl is less electronegative than oxygen atomBof the -I effect of a halogen which is greater than its +R effectCof +R effect of Cl which is stronger than its -I effectDof +R effect of Cl
p-nitro phenol is stronger acid than phenol. Explain.
View SolutionExplain Why :
(i) Carboxylic acids are stronger acids than alcohols.
(ii) Carboxylic acids are stronger acids than phenols
(iii) ∝-chloropropionic acid is a stronger acid than β-chloropropionic acid.
View SolutionExplain why carboxylic acids are stronger acids than phenols.
View SolutionPhenol is more acidic than ethanol.
View SolutionWhat makes acetic acid a stronger acid than phenol?
View Solution