Phenol when it first reacts with concentrated sulphuric acid and then with concentrated nitric acid gives
A
nitrobenzene
B
2,4,6-trinitrobenzene
C
o-nitrophenol
D
p-nitrophenol
Text Solution
The correct Answer is:C
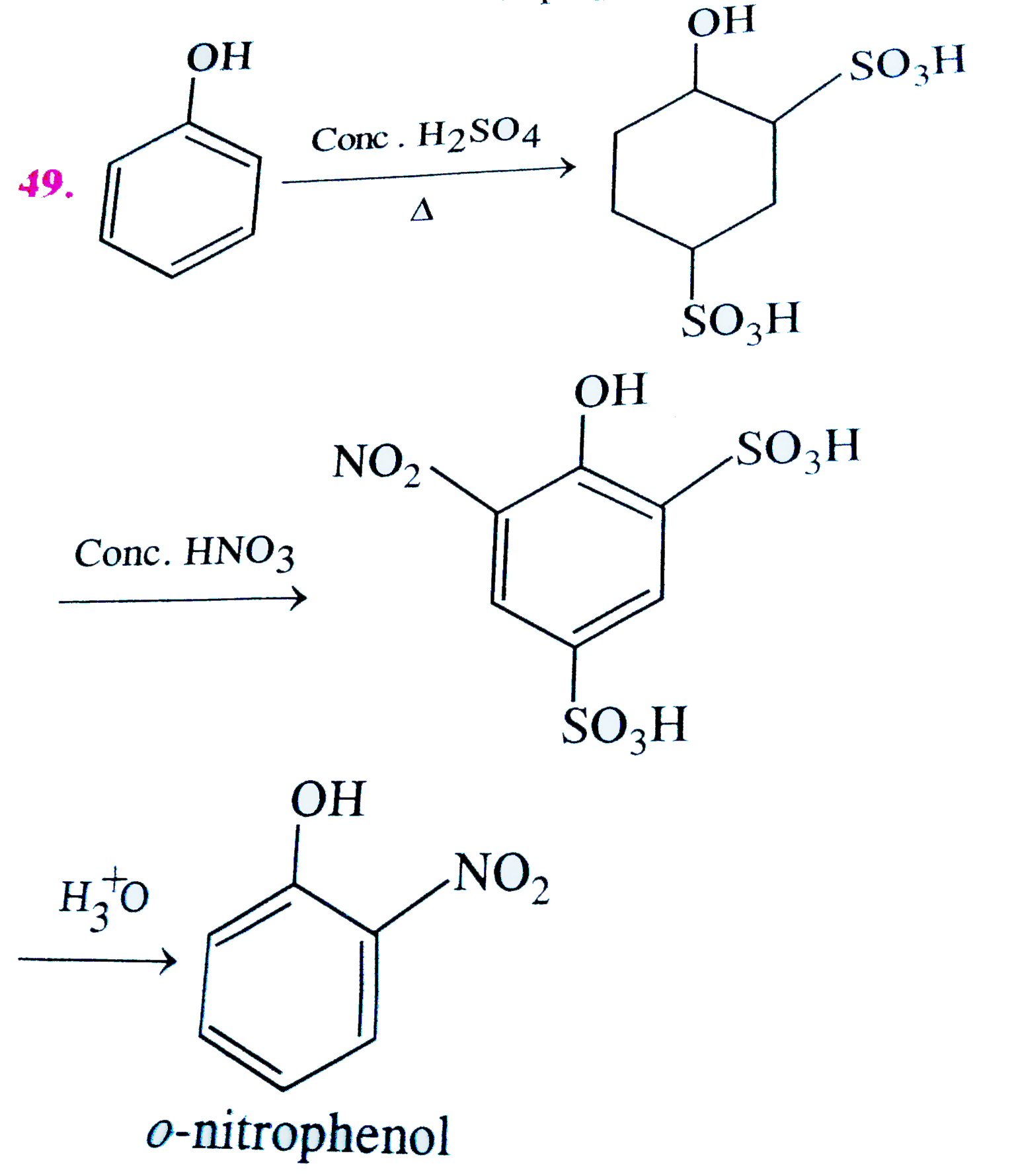
−SO3H group are knocked out on boiling with water.
|
Similar Questions
Knowledge Check
Phenol when it first reacts with concentrated sulphuric acid and then will concentrated nitric acid gives:
A2,4,6−trinitrobenzeneBpicric acidCp-nitrophenolDnirtobenzenePhenol when it first reacts with concentrated sulphuric acid and then with concentrated nitric acid gives:
A6 - trinitrotolueneBNitrophenolC2, 4, 6 0 trinitrophenolDNone of the abovePhenol , when first reacts with concentrated sulphuric acid and then when concentrated nitric acid, gives
Ao-nitrophenolBp-nitrophenolCNitrobenzeneD2,4,6-trinitrophenol
Concentrated sulphuric acid acts as
View SolutionNitration of phenol with concentrated nitric acid gives
View SolutionWhen tin is treated with concentrated nitric acid
View SolutionWhich one of the following reacts with concentrated sulphuric acid
View SolutionThe gas liberated when concentrated sulphuric acid is reated with copper
View Solution

