A is a higher phenol and B is an aromatic carboxylic acid. Separation of a mixture of A and B can be carried out easily by having a solution
NaOH
Na3CO3
Lime
NaHCO3
The correct Answer is:D
Phenol does not react with NaHCO3 whereas carboxylic acid forms sodium carboxylate
Topper's Solved these Questions
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:DINESH PUBLICATIONChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Selected straight objective type MCQs
Explore 43 Videos
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:DINESH PUBLICATIONChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Matrix Match type MCQs
Explore 2 Videos
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:DINESH PUBLICATIONChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Unit test
Explore 20 Videos
- BIOMOLECULESBook:DINESH PUBLICATIONChapter:BIOMOLECULESExercise:Reason
Explore 1 Video
- CHEMICAL BONDING AND MOLECULAR STRUCTURE Book:DINESH PUBLICATIONChapter:CHEMICAL BONDING AND MOLECULAR STRUCTURE Exercise:COMPREHENSION
Explore 4 Videos
Similar Questions
The acids which do not contain a −COOH group are
View Solution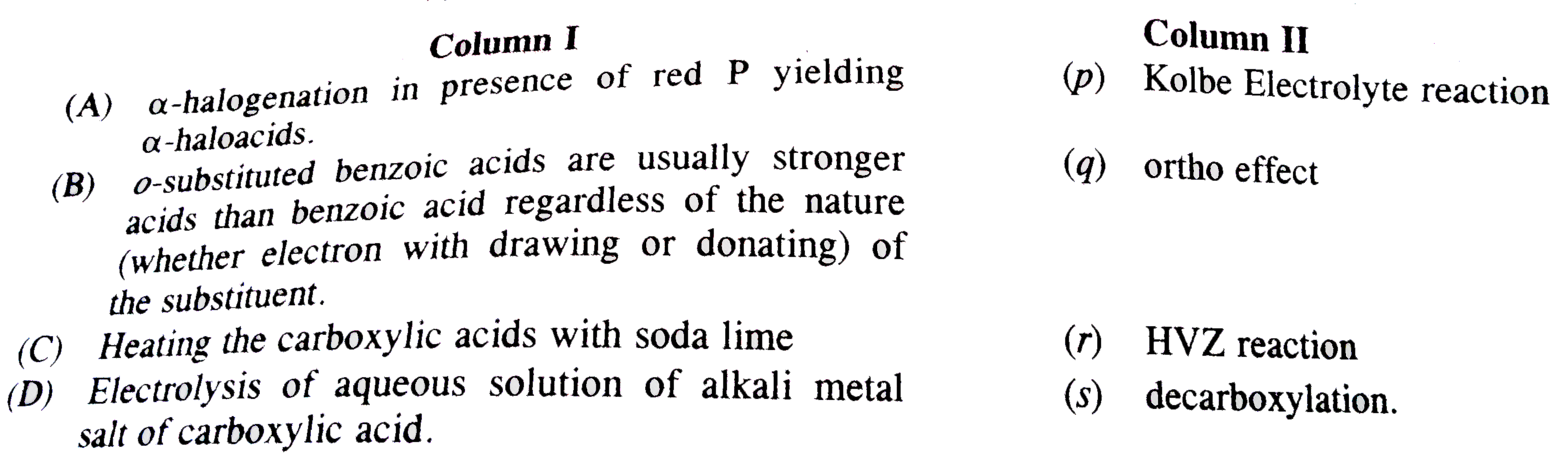
View SolutionSelect incorrect statement
View SolutionAssertion: D-mannose is the C2 -epimer of glucose.
Reason: Glucose and D-mannose differ in configuration at C2.
View SolutionMolecular orbitals are formed by the overlap of atomic orbitals. Two atomic orbitals combine atom from two molecular orbitals called vonding molecular orbital (BOM) and anti bonding molecular orbital (ABMO). Energy of anti bonding orbital is raised above the parent atomic orbitals that have combined and the energy of the bonding orbital is lowered than the parent atomic orbitals. Energies of various molecular orbitals for elements hydrogen to nitrogne increase in the order :
σ1s<σ∗1s<σ2s<σ∗2s<(π2py≈π2py)<σ2py<(π∗2py≈π∗2py)<σ∗2pz and for ozygen and fluorine order of energy of molecular orbitals id given below :
σ1s<σ∗1s<σ2s<σ∗2s<σ2pz<(π∗2px≈π∗2py)σ∗2pz
Different atomic orbitals of one atom combine wiht the atomic orbitals of the second atom whihc have comparable energies and proper orientation. Further, if the overlapping is head on, the molecular orbital is called 'Sigma' (σ) and if the overlap is atreal, the molecular orbital is called 'pi', (π). The molecular orbitals are filled with electrons according to the same rules as followed for filling of atomic orbitals. However, the order for filling is not the same for all molecules or their ions, Bond order is one the most ipmrtaint parameters to compare the strength of bonds.
Which of the following statements is correct ?
View SolutionWhich of the following reagents can be used to separate a mixture of phenol and carboxylic acid?
View SolutionThe reaction whereby a carboxylic acid loses CO_(2) is called decarboxylation. It is carried out by heating a mixture of the sodium salt of carboxylic acid and
View SolutionPhenol and carboxylic acid can be distinguished by
View Solution(A) is a higher phenol and (B) is an aromatic carboxylic acid. Separation of a mixture of (A) and (B) can be carried out easily by using a solution of:
View SolutionA is a lighter phenol and B is an aromatic carboxylic acid . Separation of a mixture of A and B can be carried out easily by using a solution of
View SolutionHow can phenol be distinguished from carboxylic acid ?
View Solutionयद्पि फिनॉक्साइड आयन की अनुनादी संरचानए कार्बोक्सिलेट आयन की तुलना में अधिक है परन्तु कार्बोक्स्लीक अम्ल फिनोल की अपेक्षा प्रबल अम्ल है । क्यों ?
View Solutionएक मिश्रण में उपस्थित कपूर तथा बेन्जोइक अम्ल को आसानी से पृथक किया जा सकता है-
View SolutionA mixture of phenol and aromatic carboxylic acid can be separated easily by using a solution of :
View SolutionA एक हल्की फीनॉल तथा B एक ऐरोमैटिक कार्बोक्सिलिक अम्ल है। A तथा B को अलग करने के लिए निम्नलिखित में से किस के विलयन का उपयोग किया जाता है?
View Solution

