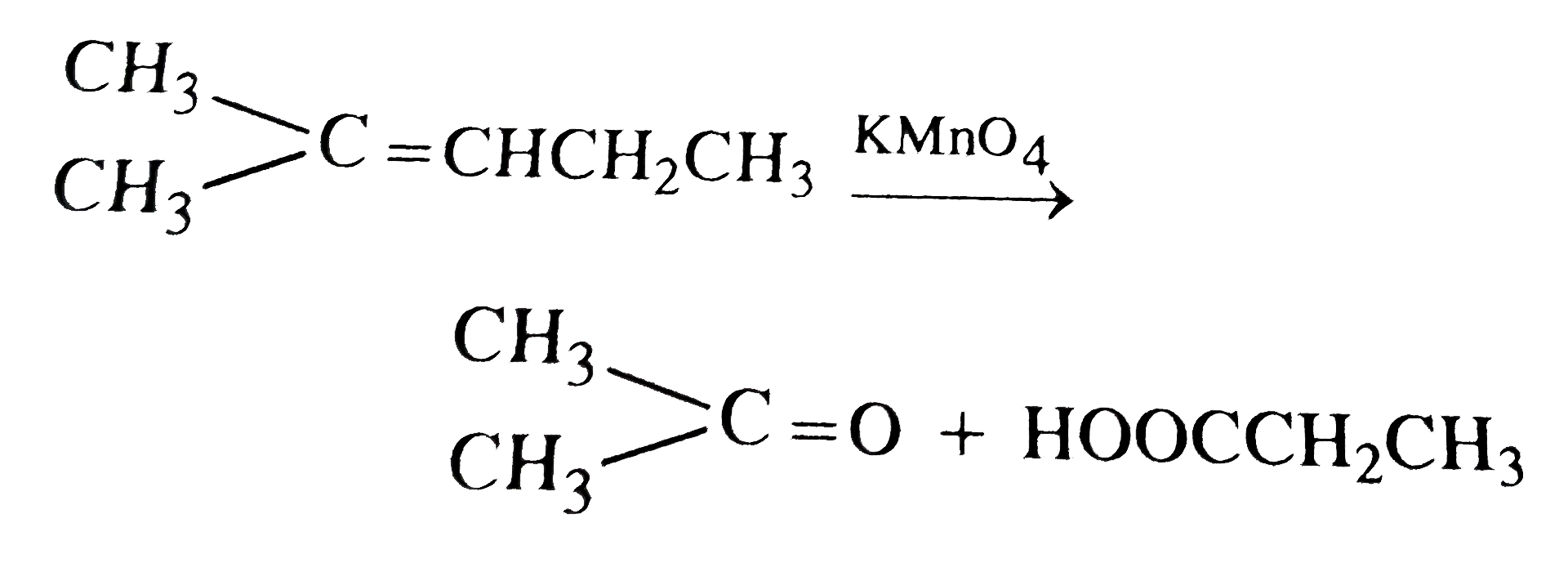On vigorous oxidation by permanganate solution (CH3)2C=CH−CH2CH3 gives
A
CH3−OH∣C∣CH3−OH∣CH−CH2CH3
B

C
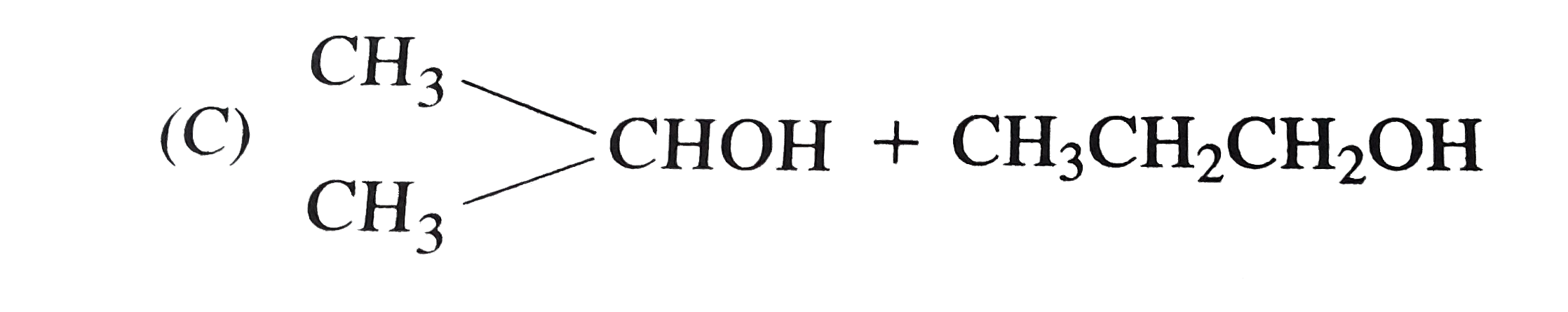
D
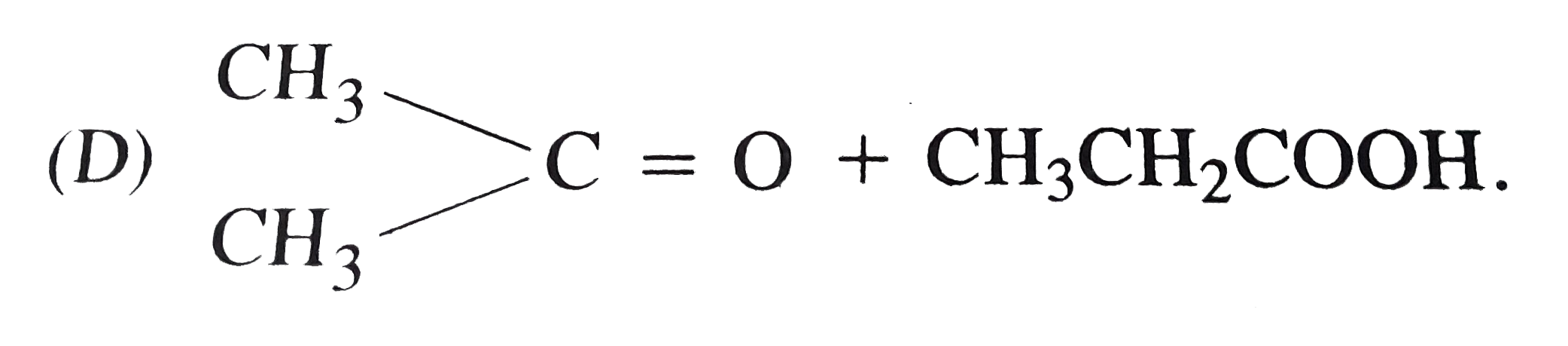
Text Solution
The correct Answer is:D
|
Similar Questions
Knowledge Check
On vigorous oxidaiton by permanganate solution (CH3)2C=CHCH2CHO gives
A(CH3)2CO and OHCCH2CHOB(CH3)2C∣OH−CH∣OH−CH2CHOC(CH3)2CO and OHCCH2COOHD(CH3)2CO and CH2(COOH)2On vigrous oxidation by permangnate solution, (CH3)2C=CH−CH2CH3 gives :
ACH3−OH∣C∣CH3−OH∣CH−CH2CH3B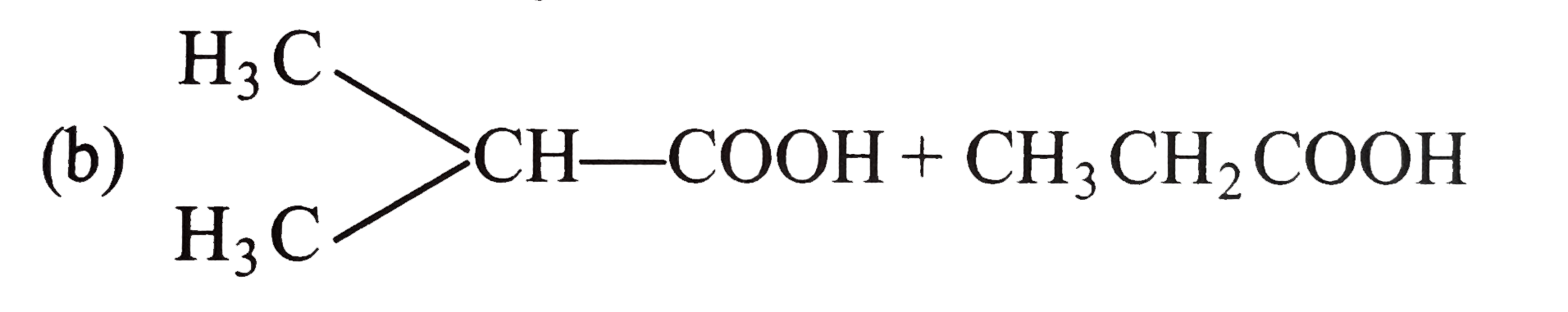 C
C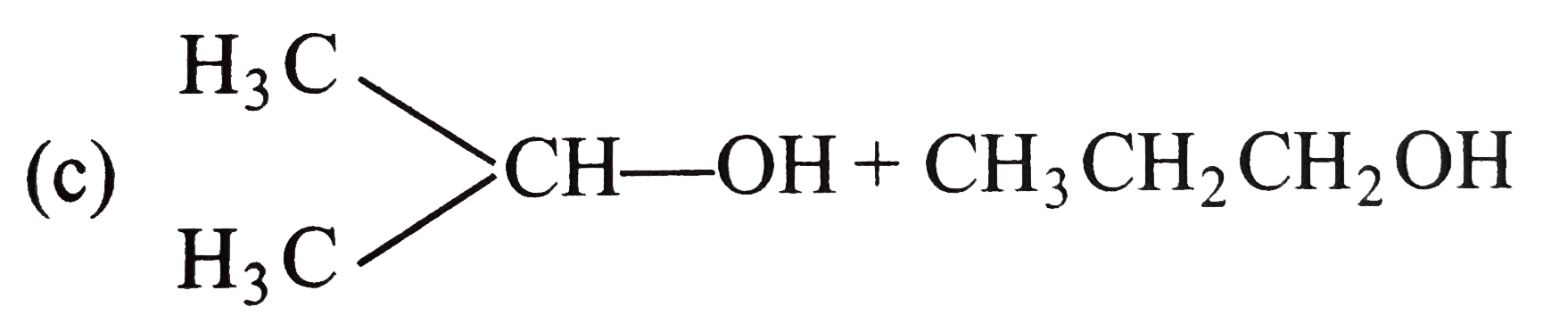 D
D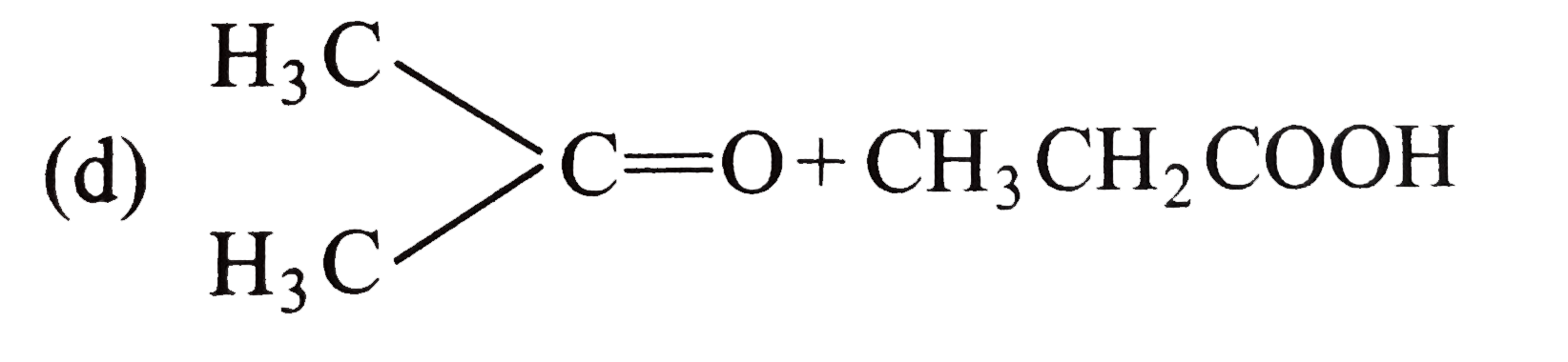
On vigorous oxidation by permangnate solution (CH3)2=CHCH2CHO gives
A(CH3)2CO and OHCCH2OCHOB(CH3)2C∣OH−C∣OHHCH2CHOC(CH3)2CO and OHCCH2COOHD(CH3)2CO and CH2(COOH)2
identify oxidation and reduction process for the reaction
CH2=CH2+H2→CH3−CH3
View SolutionGive IUPAC name of the following: CH3−CH2−CH2−C≡C−CH3
View SolutionThe number of alcohols giving iodoform test among the following is:
CH3CH2OH,CH3OH,CH3CH2CH2OH,(CH3)2(CHOH),CH3CH3CH2CH2OH
CH3CH2CH(OH)CH3,CH3CH(OH)CH(CH3)2(C2H5)2CHOH,(CH3)3COH
View SolutionAn alcohol on vigorous oxidation with K2Cr2O7 and H2SO4 gives CH3COOH and CH3CH2COOH. The alcohol is likely to be
View SolutionThe reaction of (CH3)2C(OH)CH2CH3 with copper at 300∘C gives
View Solution