Among the following acids which has the lowest pKa value ?
A
CH3COOH
B
HCOOH
C
(CH3)2CH−COOH
D
CH3CH2COOH
Text Solution
The correct Answer is:B
HCOOH is stronest acid and hence has highest Ka value and lowest pKa value
|
Similar Questions
Knowledge Check
Among the following acid which has the lowest pKa value ?
ACH3CH3OHB(CH3)2CH−COOHCHCOOHDCH3COOHAmong the following acids which has the lowest pKa value ?
A(CH3)2CH−COOHBCH3CH2COOHCCH3COOHDHCOOHwhich has the highest pKa value ?
A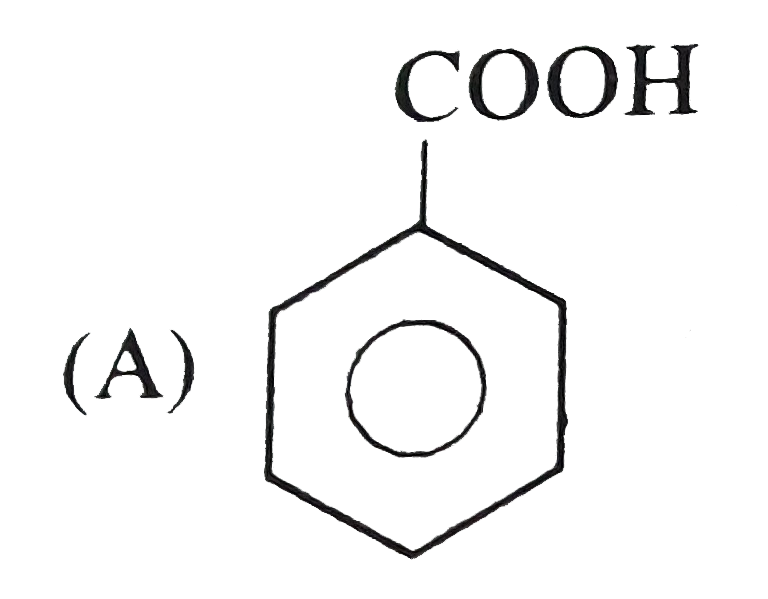 B
B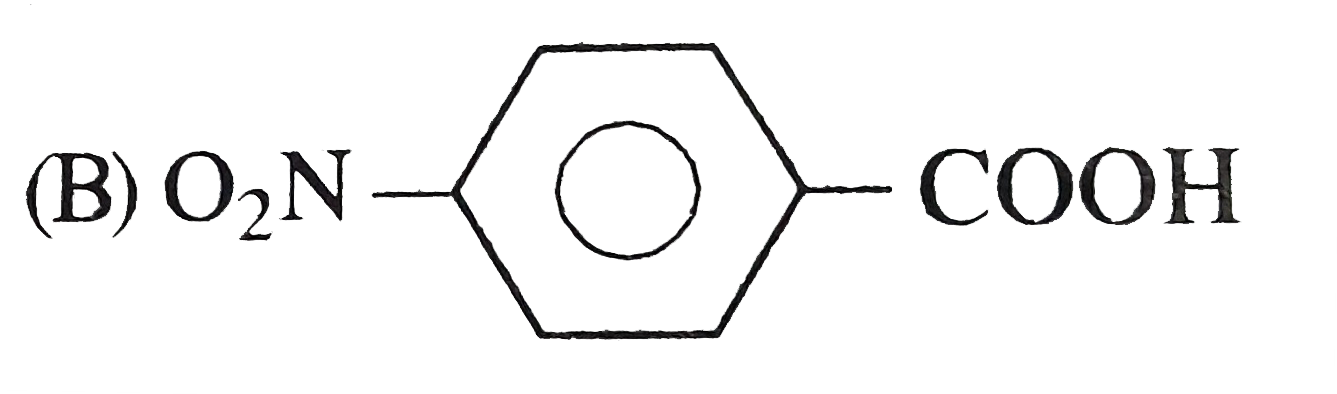 C
C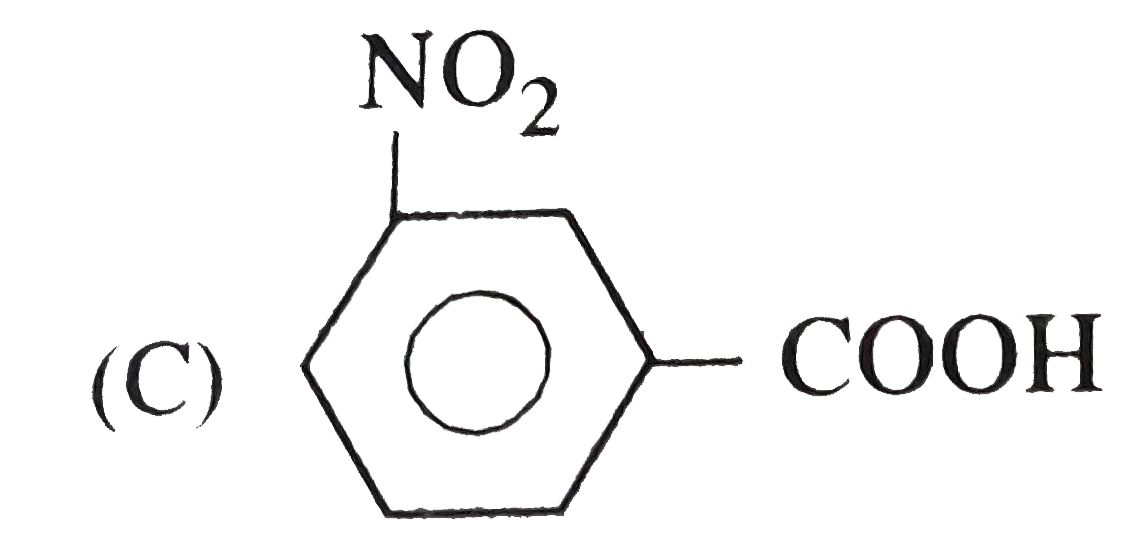 D
D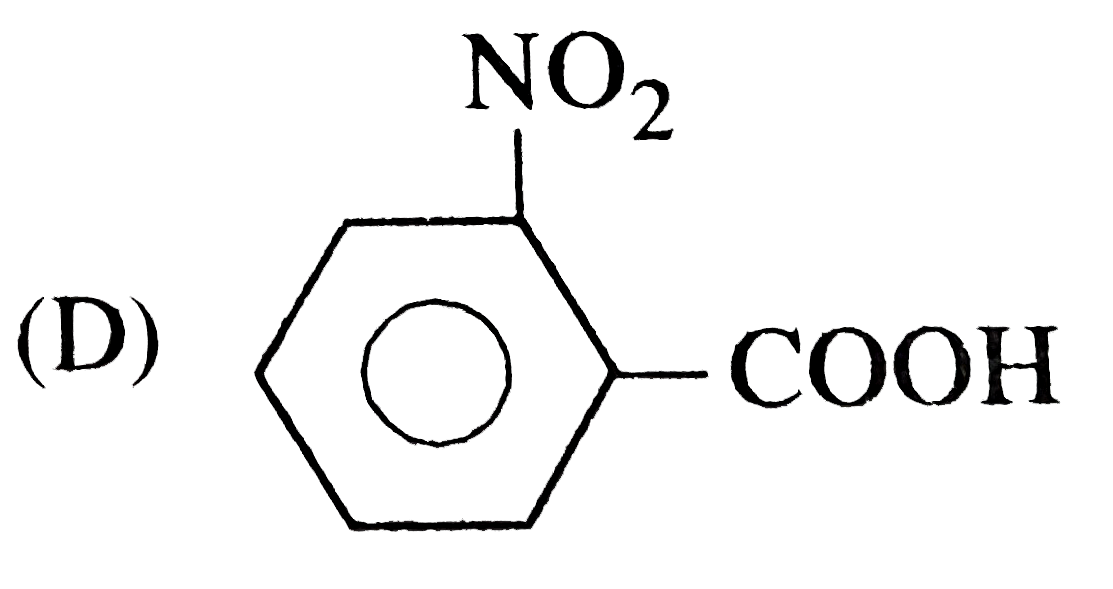
Among the acids which have lowest pKa value ?
View SolutionWhich of the following acids have the lowest pKa value :-
View SolutionAmong the following, the species which has the lowest value of bond angle is :
View SolutionWhich acid has lowest pKa
View SolutionThe base with lowest pKa value is
View Solution

