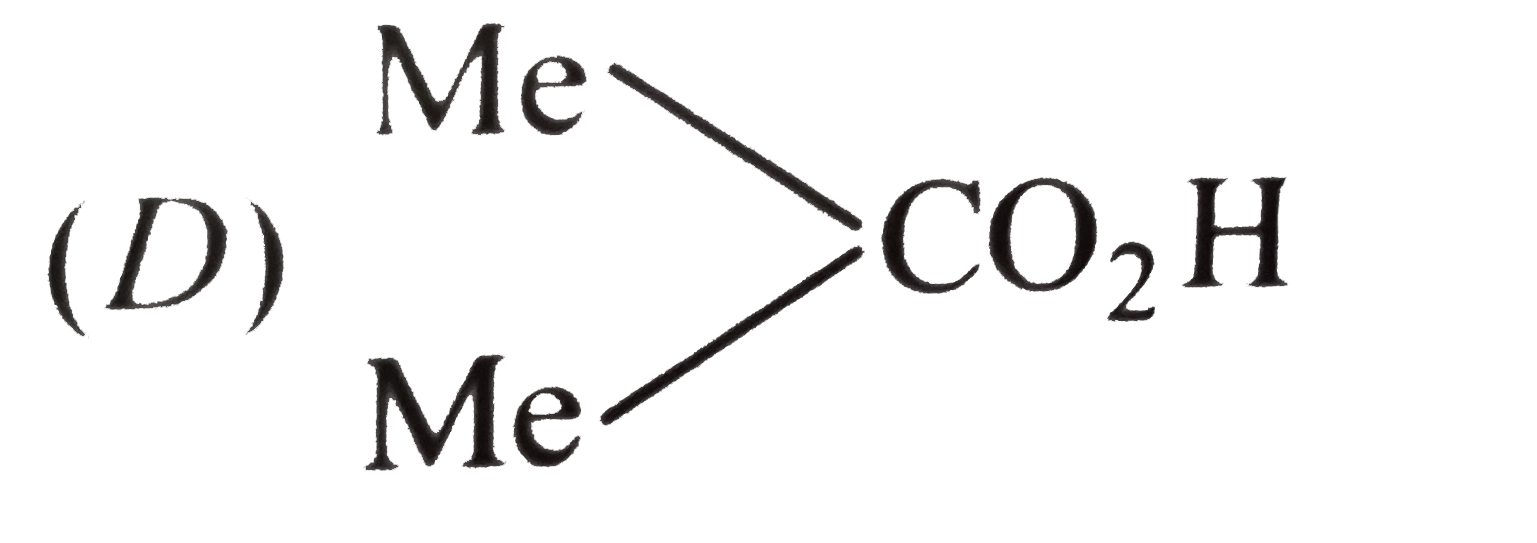
The correct order of increasing acid strength of the compounds
(a) CH3COOH
(b) MeO−CH2CO2H
(c) CF3CO2H
(d) 
A
b<d<a<c
B
d<a<c<b
C
d<a<b<c
D
a<d<c<b
Video Solution
Text Solution
The correct Answer is:C
The correct order of acid strength is

|
Similar Questions
Knowledge Check
The correct order of increasing acid strength of the compounds is
(a). CH3CO2H
(b). MeOCH2CO2H
(c) CF3CO2H
(d) Ad<a<c<bBd<a<b<cCa<d<c<bDb<d<a<c
Ad<a<c<bBd<a<b<cCa<d<c<bDb<d<a<cThe correct order of increasing acid strength of the compounds.
(a)CH3CO2H (b)MeOCH2CO2H (c)CF3CO2H (d)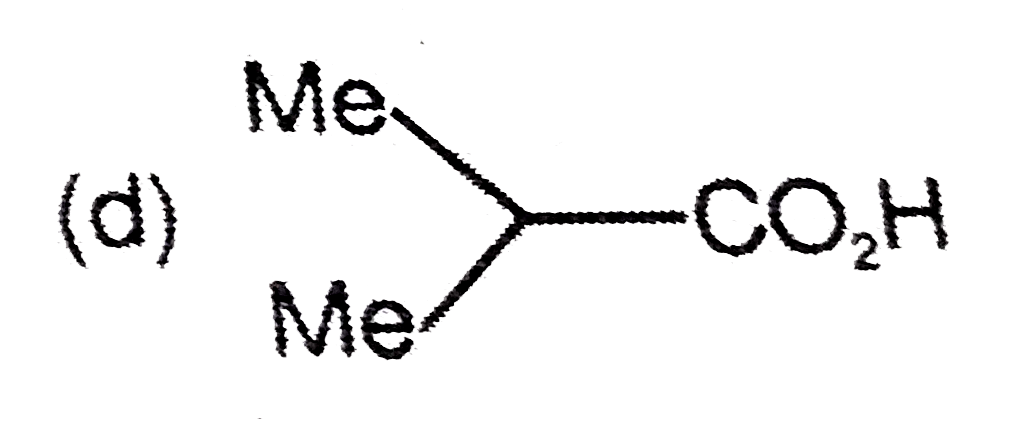 Ab<d<a<cBd<a<c<dCd<a<b<cDa<d<c<b
Ab<d<a<cBd<a<c<dCd<a<b<cDa<d<c<bThe correct order of increasing acid strength of the compounds
(a)CH3CO2H (b)MeOCH2CO2H
(c)CF3CO2H
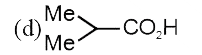 Ad<a<c<dBd<a<b≤cCa<d<c<bDb<d<a<c
Ad<a<c<dBd<a<b≤cCa<d<c<bDb<d<a<c
The correct order of increasing strength of the compounds
(a) CH3CO2H (b) MeOCH2CO2H
(c) CF3CO2H (d)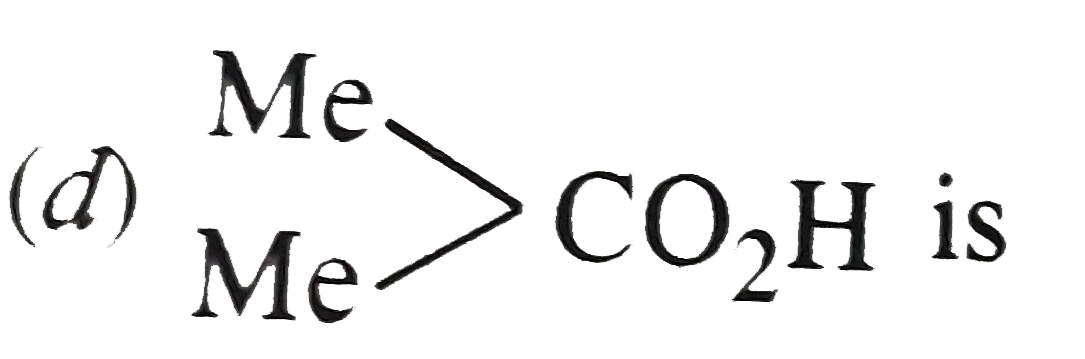
View SolutionThe correct order of increasing strength of compounds
(1) CH3COOH (2)NO2CH2COOH
(3) CF3COOH (4)C2H5−COOH
View SolutionThe correct order for the acidic strength of thhe following compounds is
I. CH3COOH II. MeOCH2COOH
III. CF3COOH IV. Me2CHCOOH
View SolutionThe correct order of acidic strength of the following compound is :
(I)CH3CH2CH2COOH(ii)C∣CNH2−COOH
(III)H2C=CH−CH2COOH
View SolutionThe correct order of acid strength of compounds CH≡CH,CH3−C≡CH and CH2=CH2 is as follows :
View Solution