In H3O+ the formal charge on the oxygen atom is :
+1
−1
zero
+2
The correct Answer is:A
To find the formal charge on the oxygen atom in the ion H3O+, we can follow these steps:
Step 1: Determine the number of valence electrons for oxygen.
Oxygen is in group 16 of the periodic table and has 6 valence electrons.
Hint: Remember that the group number for main group elements gives you the number of valence electrons.
Step 2: Identify the number of non-bonding electrons on oxygen.
In H3O+, the oxygen atom has 2 non-bonding electrons (these are the lone pair electrons).
Hint: Non-bonding electrons are those that are not involved in bonding with other atoms.
Step 3: Count the number of shared (bonding) electrons.
In H3O+, oxygen forms three bonds with hydrogen atoms. Each bond consists of 2 electrons, so the total number of shared electrons is 3×2=6 electrons.
Hint: Each bond between oxygen and hydrogen contributes 2 electrons to the shared count.
Step 4: Apply the formal charge formula.
The formal charge (FC) can be calculated using the formula:
FC=Valence electrons−Non-bonding electrons−12×Shared electrons
Substituting the values we have:
FC=6−2−12×6
Step 5: Calculate the formal charge.
Now, simplify the expression:
FC=6−2−3=1
Conclusion:
The formal charge on the oxygen atom in H3O+ is +1.
Final Answer: The formal charge on the oxygen atom is +1.
---
Oxygen is in group 16 of the periodic table and has 6 valence electrons.
In H3O+, the oxygen atom has 2 non-bonding electrons (these are the lone pair electrons).
In H3O+, oxygen forms three bonds with hydrogen atoms. Each bond consists of 2 electrons, so the total number of shared electrons is 3×2=6 electrons.
The formal charge (FC) can be calculated using the formula:
FC=Valence electrons−Non-bonding electrons−12×Shared electrons
Substituting the values we have:
FC=6−2−12×6
Now, simplify the expression:
FC=6−2−3=1
The formal charge on the oxygen atom in H3O+ is +1.
Similar Questions
The formal charge on the triply bonded oxygen atom in the structure :O≡C−⋅⋅O⋅⋅: is ……………..
View SolutionIn NO−3 ion the formal charge on the oxygen atom of N−O bond is
View SolutionFormal charge on two O atoms in
View SolutionThe formal charges on the three oxygen atoms in O_3 , molecule are
View SolutionIn
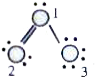 molecule, the formal charges of oxygen atoms 1,2,3 are respectively
molecule, the formal charges of oxygen atoms 1,2,3 are respectively
View SolutionIn PO_4(3-) ion, the formal charge on the oxygen atom of P-O bonds is
View SolutionThe formal charge on the central oxygen atom in O3 molecule is
View SolutionIn H_(3)O^(+) the formal charge on the oxygen atom is :
View Solution
