Arrange the following in increasing order of basic strength : MgO,SrO,K2O,NiO,CsO2.
To arrange the oxides MgO, SrO, K₂O, NiO, and CsO₂ in increasing order of basic strength, we need to consider the trends in basic strength of metal oxides based on their positions in the periodic table.
1. Identify the Elements and Their Groups:
- MgO: Magnesium (Mg) is in Group 2 (alkaline earth metals).
- SrO: Strontium (Sr) is also in Group 2 (alkaline earth metals).
- K₂O: Potassium (K) is in Group 1 (alkali metals).
- NiO: Nickel (Ni) is in Group 10 (transition metals).
- CsO₂: Cesium (Cs) is in Group 1 (alkali metals).
2. Understanding Basic Strength Trends:
- Basic strength of metal oxides generally increases down a group and decreases across a period.
- For alkali metals (Group 1), the basic strength increases down the group (Li < Na < K < Rb < Cs).
- For alkaline earth metals (Group 2), the basic strength also increases down the group (Be < Mg < Ca < Sr < Ba).
- Transition metals (like Ni) typically have lower basic strength compared to alkali and alkaline earth metals.
3. Rank the Oxides Based on Basic Strength:
- NiO: Being a transition metal oxide, it has the least basic strength.
- MgO: As a Group 2 oxide, it has moderate basic strength.
- SrO: Also a Group 2 oxide but lower than MgO due to being further down the group.
- K₂O: As a Group 1 oxide, it has higher basic strength than MgO and SrO.
- CsO₂: As a Group 1 oxide, it has the highest basic strength due to being the lowest in the group.
4. Final Arrangement in Increasing Order of Basic Strength:
- NiO < MgO < SrO < K₂O < CsO₂
Conclusion:
The increasing order of basic strength for the given oxides is:
NiO < MgO < SrO < K₂O < CsO₂
- MgO: Magnesium (Mg) is in Group 2 (alkaline earth metals).
- SrO: Strontium (Sr) is also in Group 2 (alkaline earth metals).
- K₂O: Potassium (K) is in Group 1 (alkali metals).
- NiO: Nickel (Ni) is in Group 10 (transition metals).
- CsO₂: Cesium (Cs) is in Group 1 (alkali metals).
- Basic strength of metal oxides generally increases down a group and decreases across a period.
- For alkali metals (Group 1), the basic strength increases down the group (Li < Na < K < Rb < Cs).
- For alkaline earth metals (Group 2), the basic strength also increases down the group (Be < Mg < Ca < Sr < Ba).
- Transition metals (like Ni) typically have lower basic strength compared to alkali and alkaline earth metals.
- NiO: Being a transition metal oxide, it has the least basic strength.
- MgO: As a Group 2 oxide, it has moderate basic strength.
- SrO: Also a Group 2 oxide but lower than MgO due to being further down the group.
- K₂O: As a Group 1 oxide, it has higher basic strength than MgO and SrO.
- CsO₂: As a Group 1 oxide, it has the highest basic strength due to being the lowest in the group.
- NiO < MgO < SrO < K₂O < CsO₂
The increasing order of basic strength for the given oxides is:
NiO < MgO < SrO < K₂O < CsO₂
Similar Questions
Knowledge Check
Arrange the following in increasing order of acidic strength
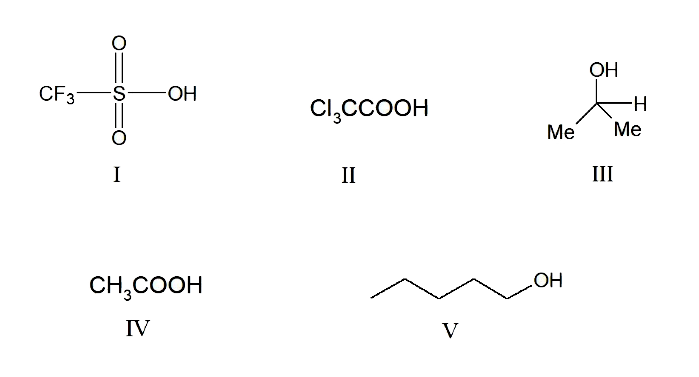 AIII < V < IV < II < IBV < IV < III < II < ICIII < II < IV < I < VDI < II < III < IV < V
AIII < V < IV < II < IBV < IV < III < II < ICIII < II < IV < I < VDI < II < III < IV < VArrange the following in increasing order of basicity.
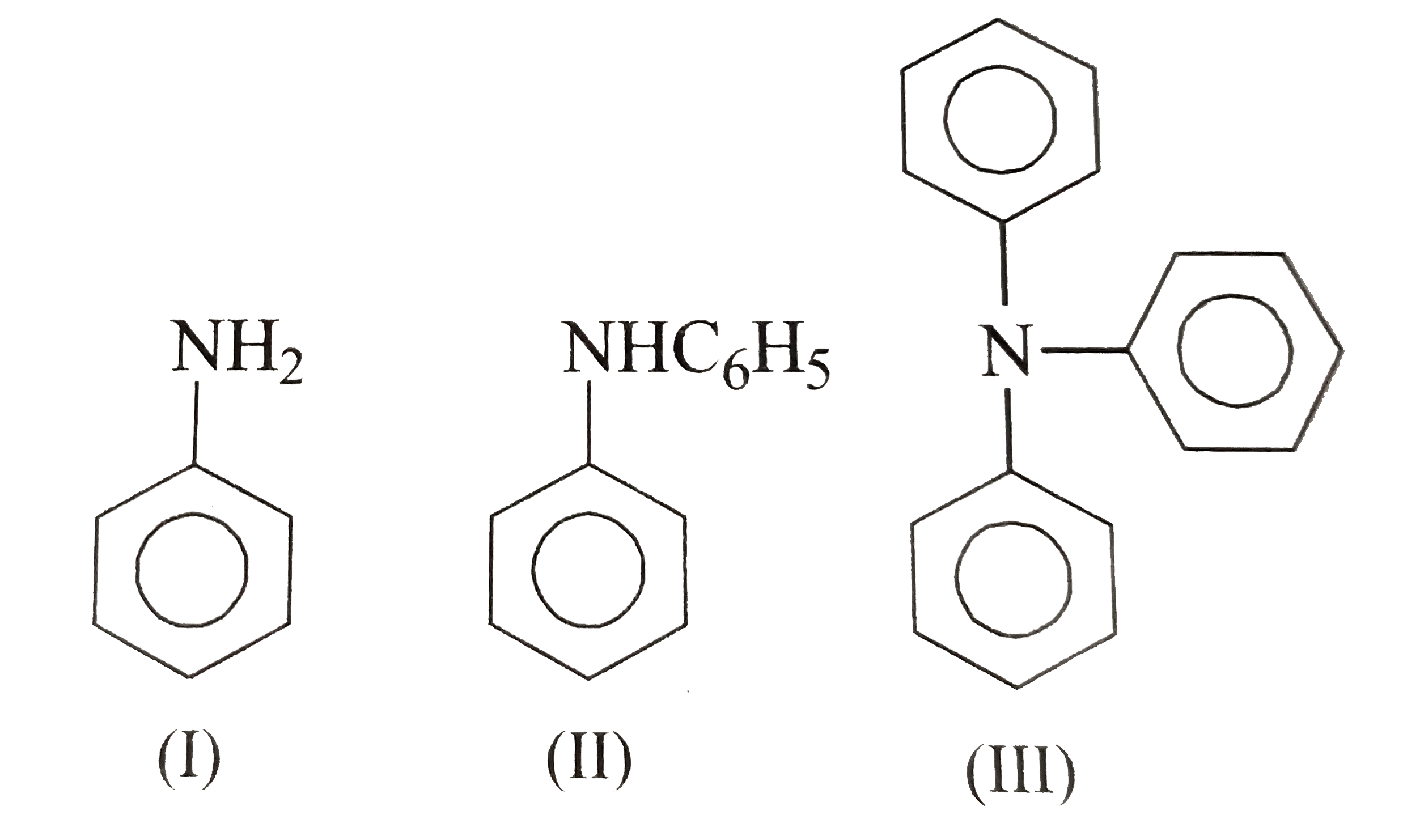 AI<II<IIIBIII<I<IICIII<II<IDII<III<I
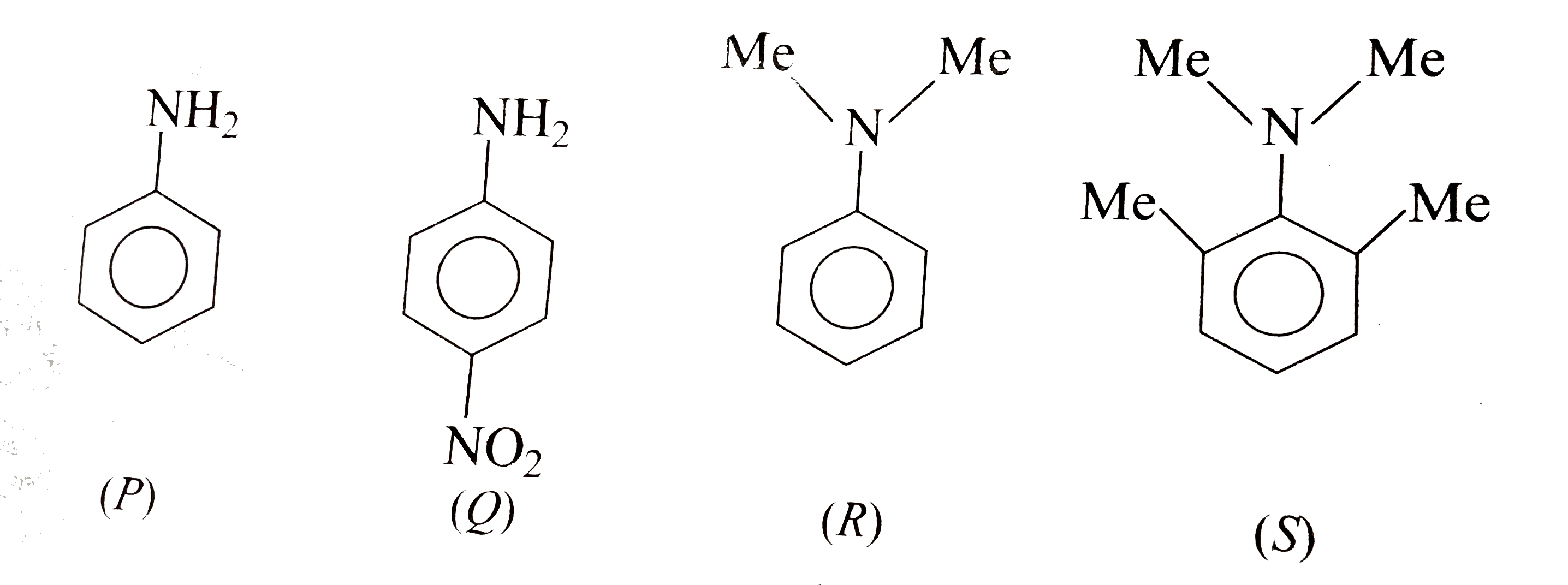
AI<II<IIIBIII<I<IICIII<II<IDII<III<IArrange the following in increasing order of basicity :
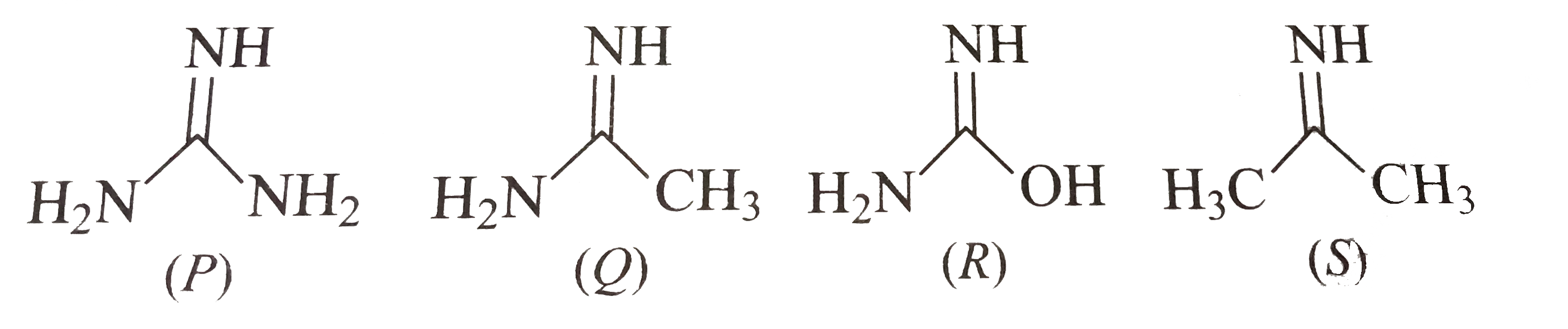 AS<Q<R<PBS<Q<P<RCS<R<P<QDP<Q<R<S
AS<Q<R<PBS<Q<P<RCS<R<P<QDP<Q<R<S
Arrange the following bases in increasing order of their basic strength
NH4OH,NaOH,Mg(OH)2,AI(OH)3
View SolutionArrange the following in order of increasing basic strength.
View SolutionArrange the following in the increasing order of acidic strength
(i) FSO3H (ii) HCO−3 (iii) H2O (iv) HSO−4
View SolutionArrange the following compounds in increasing order of acid strength
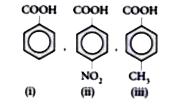
View SolutionArrange the following compounds in increasing order of basicity :
View Solution

