Which of the following statements are correct about solid catalyst?
A
Same reactants may give different products by using different catalysts
B
Catalyst does not change ΔH of reaction
C
Catalyst is required in large quantities to catalyse reactions
D
Catalytic activity of a solid catalyst does not depend upon the strength of chemisorption
Video Solution
Text Solution
The correct Answer is:A, B
(a) Same reactants may give different products by using different catalysts as different catalysts have different specific functions to mold the reaction towards specific product.
e.g., starting with H2 and CO, and using different catalysts, we get different products.
(i) CO(g)+3H2Ni−−→CH4(g)+H2O(g)
(ii) CO(g)+2H2(g)Cu/ZnO−−−−−−−→Cr2O3CH3OH(g)
(iii) CO(g)+H2(g)Cu−−→HCHO(g)
(b) Catalyst does not change ΔH of reaction as ΔH of reaction is difference between enthalpy of reactants and products. So, it does not change during catalysed reaction.
|
Similar Questions
Knowledge Check
Which of the following statement is correct about sulphur
AAt 160∘C, the yellow liquid of sulphur becomes dark brownBIt melts at 115∘C and forms pale yellow liquidCIt boils at 444∘C giving away yellow brown vapoursDAll of theseWhich of the following statements are correct about halr-period ?
AIt is proportional to intial concentration for zeroth orderBAverage life =1.44× half-life for first order reaction.CTiem of 75% comletion of reaction is thrice of halr-life (intial harl-life) period in second order reactionD99.9% reaction takes place in 100 minutes for the case when reate constant is 0.693−1minWhich of the following statements are correct about the diamagnetic solids ?
AThey have only paired electronsBThey are weakly repelled in magnetic field.CThey have large number of unpaired electronsDChromium is diamagnetic.
Which of the following statements are correct about the following reaction
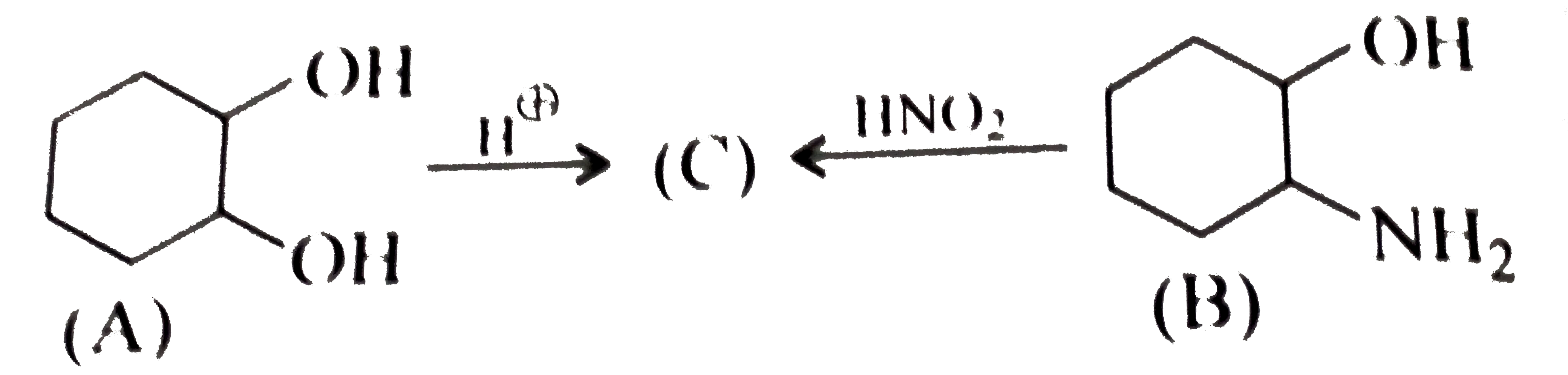
View SolutionWhich of the following statements is correct about receptors?
View SolutionWhich of the following statements is correct about receptors?
View SolutionWhich of the following statement is correct about gravity?
View SolutionWhich of the following statements is/are correct about convection?
View Solution


 .
.