Freundlich adsorption isotherm is given by the expression xm=kp1n Which of the following conclusions can be drawn from this expression?
When 1n=0, the adsorption is independent of pressure
When 1n=0, the adsorption is directly proportional to pressure
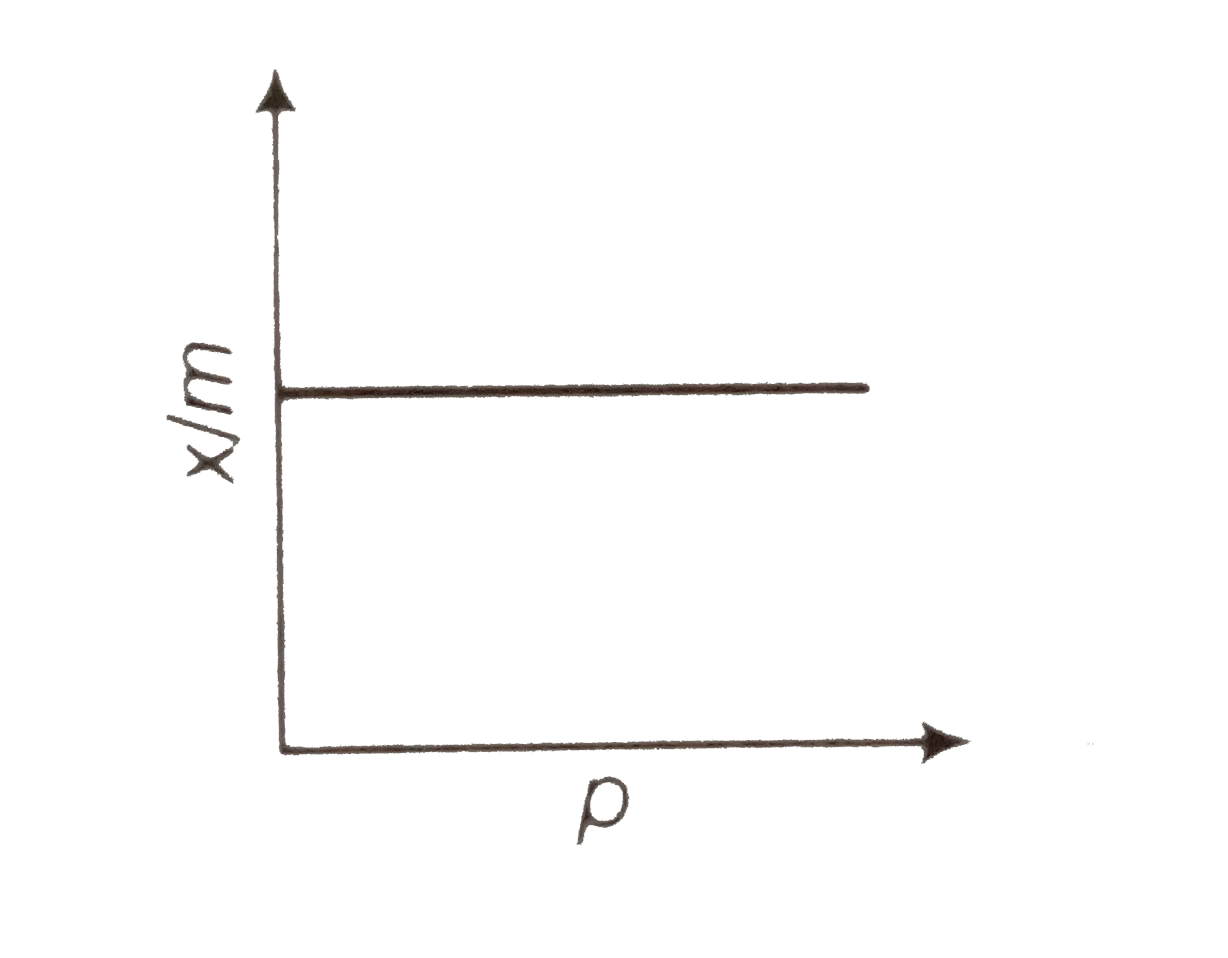
When n=0,xm vs p graph is a line parallel to x-axis
When n=0, plot of xm vs p is a curve
The correct Answer is:A, C
According to Freundlich adsorption isotherm
xm∝p1n⇒xm=kp1n
(a) At 1n=0 this equation becomes xm=kp∘=k
Extent of adsorption is independent of pressure.
(c) At x=0, xm=kp10=kp∞
Hence, xm vs p graph can be plotted as
Similar Questions
Freundlich adsorption isotherm is given by the expression x//m=kp^(1//n) which of the following conclusions can be drawn from this expression.
View SolutionFreundlich adsorption isotherm is given by the expression (x)/(m) = kP^(1//n) . Then the slope of the line in the following plot is
View SolutionFreundlich adsorption isotherm is given by the expression xm=kp1/n. Then the slope of the line in the following plot is

View SolutionFreundlich adsorption isotherm is given by the expression xm=kp1n. Which of the following conclusions can be drawn from this expression ?
View SolutionFreundlich adsorption isotherm is given by the expression (x)/(m) = kp^((1)/(n)) . Then the slope of the line in the following plot is
View SolutionFor Freundlich adsorption isotherm, (x)/(m)=kp^1//n , the value of n is
View Solutionफ्रॉयन्डलिक अधिशोषण समतापी को xm=kp1n व्यंजक द्वारा दिया जाता है। इस व्यंजक से निम्नलिखित में से कौन-से परिणाम निकलते हैं?
View SolutionFreundlich adsorption isotherm is xm=kp1n where n>1. Anser the following questions based on Freundlich adsorption isotherm: What is adsorption isotherm?
View Solution
