What is meant by hybridisation ? Compound CH2=C=CH2 contains sp or sp2 hybridised carbon atoms. Will it be a planar molecule ?
Video Solution
Text Solution
Hybridisation is mixing of atomic orbitals to form new hybrid orbitals. The new orbital have the same total electron capacity as the old ones. The properties and energies of the new hybridised orbitals are an average of the unhybridised orbitals.
1CH2=2C==3CH2 3σ 2σ 3σ
Hybridisation can be found by counting numbeer of σ- bonds around the carbon atom.
3σ=sp2 hybridisation 2σ=sp hybridisation
In allene, carbon atoms 1 and 3 are sp2-hybridised as each one of them is joined by a double bond. And, carbon atom 2 is sp-hybridised as it has two double bonds at each of its side. Therefore, the two π−bonds are perpendicular to each other, in allene, as shown below.
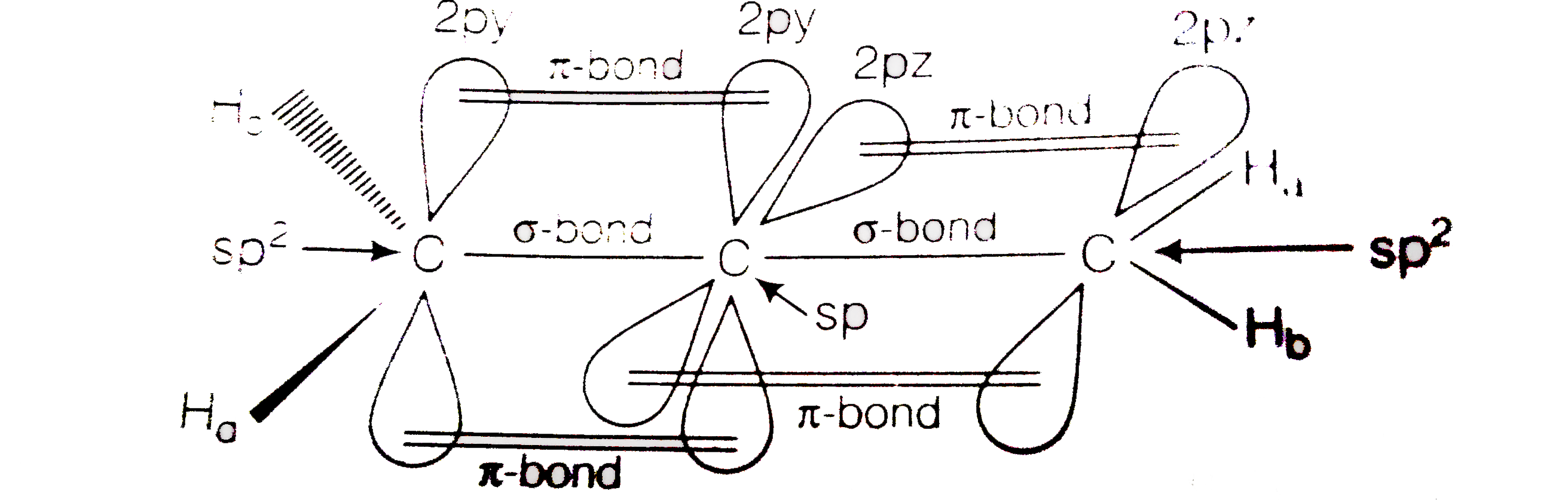
Ha and Hb lie in the plane of paper while Hc and Hd lie in a plane perpendicular to the plane of the paper. Hence, the allene molecule as a whole is non-planar.
|
Similar Questions
Knowledge Check
All carbon atoms are sp2 hybridised in
ACH2=C=CH2B1, 3- butadieneCCH≡C−C≡CHDCyclohexaneHybridisation at 2nd carbon in
CH2=CH−CH3 isAspBsp2Csp3Dsp3dAll carbon atoms are sp2-hybridised in:
AHC≡C−C≡CHBH2C=C=CH2C2-buteneD1,3-butadine
What is the hybridisation of .each carbon in CH2=CH−CH3 ?
View SolutionIn CH2=C=CH2, all the carbon atoms are sp2 hybridised.
View SolutionAssertion : In CH2=C=CH2, all the carbon atoms are sp2-hybridized.
Reason : All the hydrogen atoms lie in one plane.
View SolutionAll carbon atoms sp2 hybridised in
View SolutionAssertion : In CH2=C=CH2, all the carbon atoms are sp2-hybridized.
Reason : All the hydrogen atoms lie in one plane.
View Solution
