Write the mechanism of the reaction of HI with methoxybenzene.
Video Solution
Text Solution
In case of alkyl aryl ethers, the products are always phenol and an alkyl halide because due to resonance C6H5−O bond has partial double bond character. The mechanism is given below.
Mechanism Protonation of anisole gives methylphenyl oxonium ion.
C6H5−..O..CH3+H+→C6H5−+O∣H:−CH3
In this ion, the bond between O−CH3 is weaker than the bond between O−C6H5 which has partial double bond character. The partial double bond character is due to the resonance between the lone pair of electrons on the O atom and the sp2 hybridised carbon atom of the phenyl group. Therefore, attack by I− ion exclusively breaks the weaker O−CH3 bond forming methyl iodide and phenol.
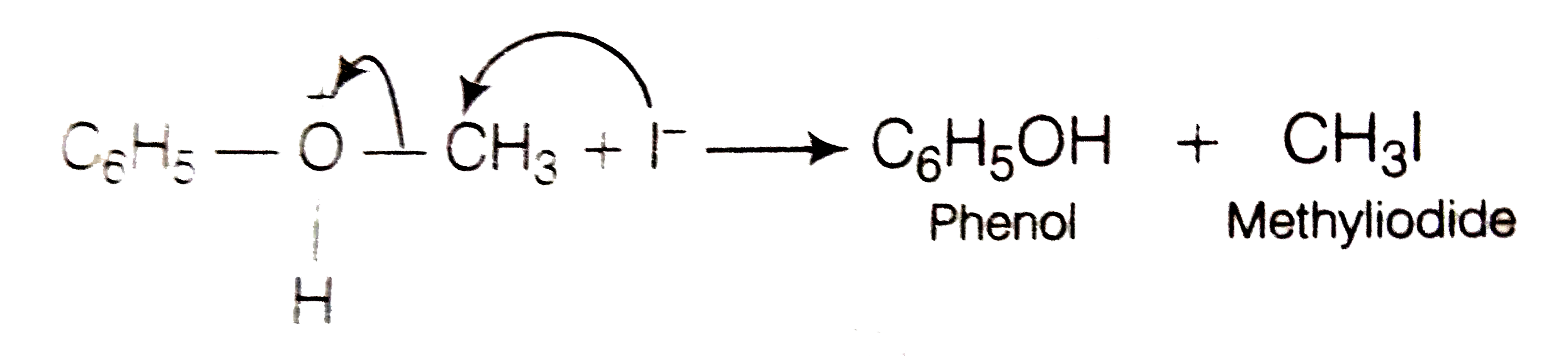
|
Similar Questions
Write the mechanism of the reaction of HI with methoxymethane.
View SolutionWrite the mechanism of the reaction of HI with methoxymethane.
View SolutionGive the mechanisms of the reaction
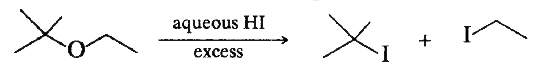
View SolutionGive the mechanisms of the reaction
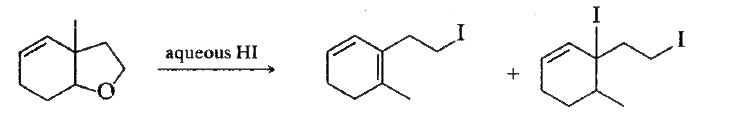
View SolutionWrite the mechanism of the reaction of Hiwith methoxymethane.
View SolutionMechanism of name reaction
View Solution_E01_072_Q01.png)
Give mechanism of this reaction.
View SolutionMechanism Of A Reaction
View SolutionWrite the mechanism of dehydration of ethanol.
View SolutionWrite the mechanism for the following reaction
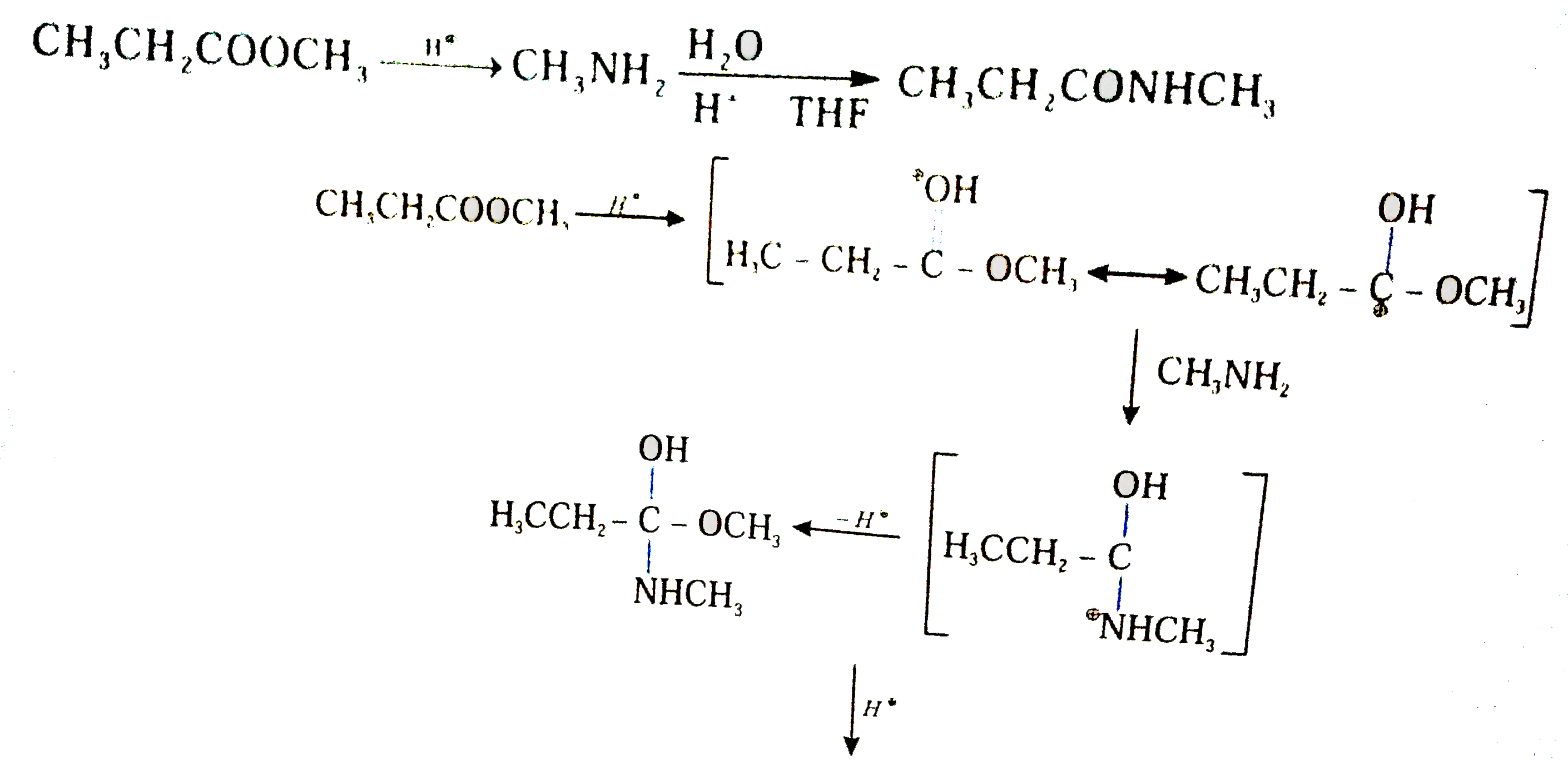
View Solution
