Give reasons for the following
(a) CCl4 is immiscible in water, whereas SiCl4 is easily hydrolysed.
(b) Carbon has a strong tendency for catenation compared to silicon.
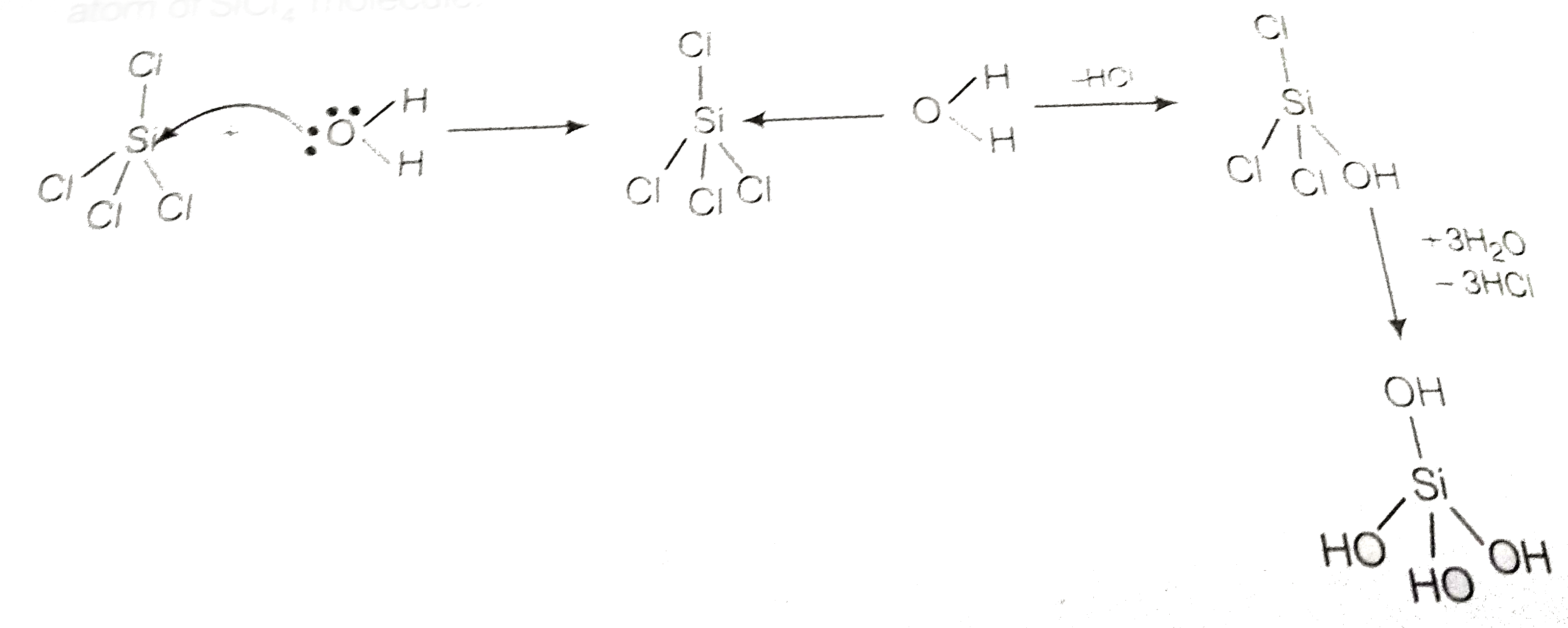
(a) Carbon tetrachloride (CCl4) is a covalent compound while H2O is a polar compound. CCl4 does not form H-bond with water molecule. Hence, it is immiscible in water. Further more, CCl4 is not hydrolysed by water because of the absence of d-orbitals in carbon while SiCl4 is readily hydrolysed by water.
SiCl4+4H2O→Si(OH)4Silicic acid+4HCl
The hydrolysis of SiCl4 occurs due to coordination of OH− with empty 3d orbitals in silicon atom of SiCl4 molecule
(b) Carbon atoms have the tendency to link with one another through covalent bonds to form chains and rings. This property is called catenation. This property is called catenation. This is because C-C bonds are very strong
Down the group, the size increases and electronegativity decreases and thereby, tendency to show catenation decreases. Thus, carbon has a strong tendency for catenation as compared to silicon.
Similar Questions
Knowledge Check
CCl4 is inert towards hydrolysis but SiCl4 is readily hydrolysed b ecause:
Acarbon cannot expand its octet but silicon can expand its octetBionisation potential of carbon is higher than siliconCcarbon forms double and triple bondsDelectronegatively of carbon is higher thatn that of silicon.CCl4 is inert towards hydrolysis but SiCl4 is readily hydrolysed because
Acarbon cannot expand its octet but silicon can expand its octetBionisation potential of carbon is higher than siliconCcarbon forms double and triple bondsDelectronegativity of carbon is higher than of siliconCCl4 does not show hydrolysis but SiCl4 is readily hydrolysed because:
AIP of carbon is higher than that of siliconBelectronegativity of carbon is higher than that of siliconCcarbon cannot expand its octate but silicon can expandDcarbon forms doyble and triple bonds but not silicon
Explain the following situations :
SF4 is easily hydrolysed whereas SF is not easily hydrolysed.
View SolutionGive reasons for the following observations :
Why SF4 undergoes hydrolysis but not SF6?
Or SF6 is inert towards hydroysis.
SF6 is much less reactive than SF4.
Or (i) SF4 is easily hydrolysed whereas SF6 is not easily hydrolysed.
(ii) Sulpher exhibits greater tendency for calenation than selenium.
(iii) Sulpher has a higher tendency for catenation than oxygen.
View SolutionSF6 is not easily hydrolysed whereas SF4 can be. Assign reason.
View SolutionState reasons for each of the following :
(i) All the P-Cl bonds in PCl5 molecule are not equivalent.
(ii) Sulphur has greater tendency for catenation than oxygen.
View SolutionThe main reason that SiCl4 is easily hydrolysed as compared to CCl4is that
View Solution
