Predict the hybridisation of each carbon in the molecule of organic compound given below. Also indicate the total number of sigma and pi-bonds in this molecule.
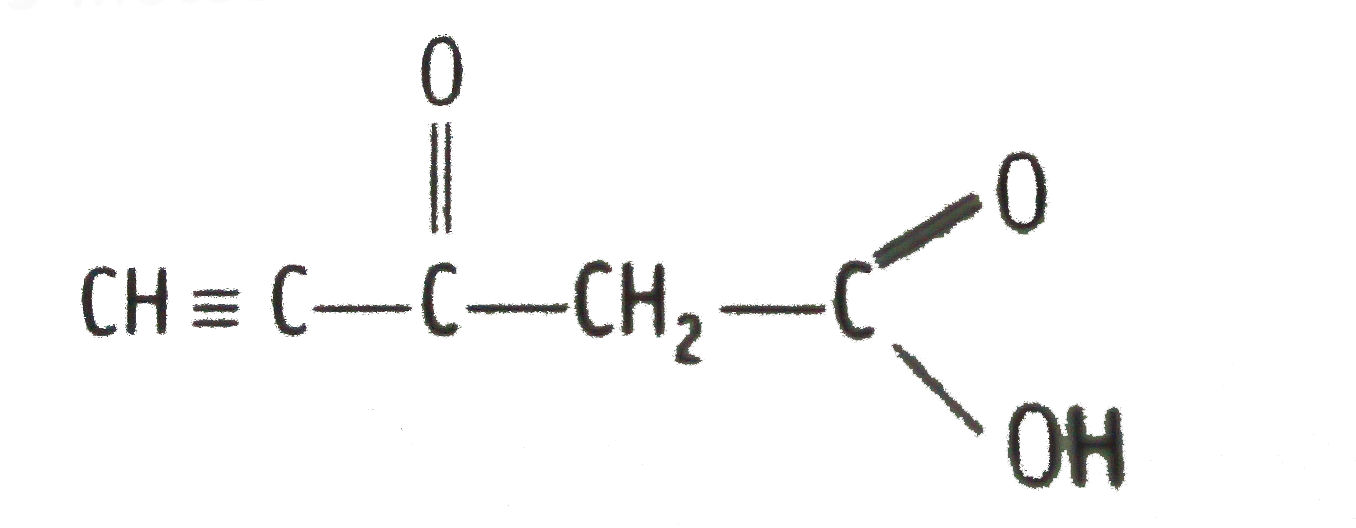
Video Solution
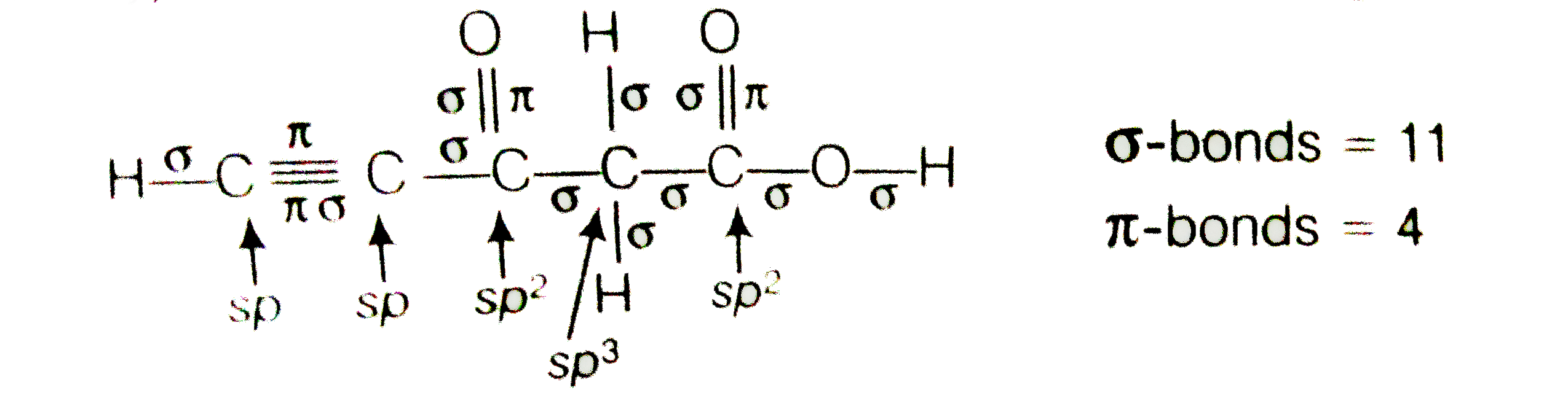
Text Solution
The hybridisation and type of bonds of each carbon in the molecule given below.
|
Similar Questions
Knowledge Check
Total number of σ and π-bonds are in naphthalene is
A5π and 18σB6π and 19σC5π and 19σD7π and 26σThe number of σ bonds in naphthalene molecule is :
A19B11C21D15Total number of sigma and π bonds present in ethyne are
A4 and 3 respectivelyB3 and 2 respectivelyC2 and 3 respectivelyD1 and 2 respectively
Write the number of σ and π bonds in the following molecules?
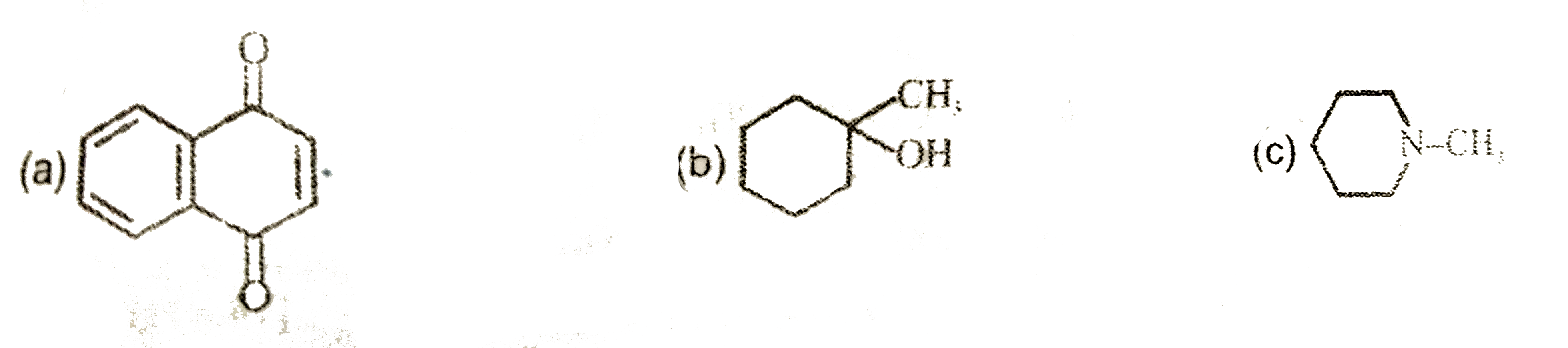
View SolutionTotal number of covalent bonds in an ethane molecule is
View SolutionThe number of sigma and Pi bonds in a molecule of cyanogen are
View SolutionThe number or pπ−dπ bonds in SO3 molecule is
View SolutionThe number σ− bond in H2 molecule is -
View Solution

