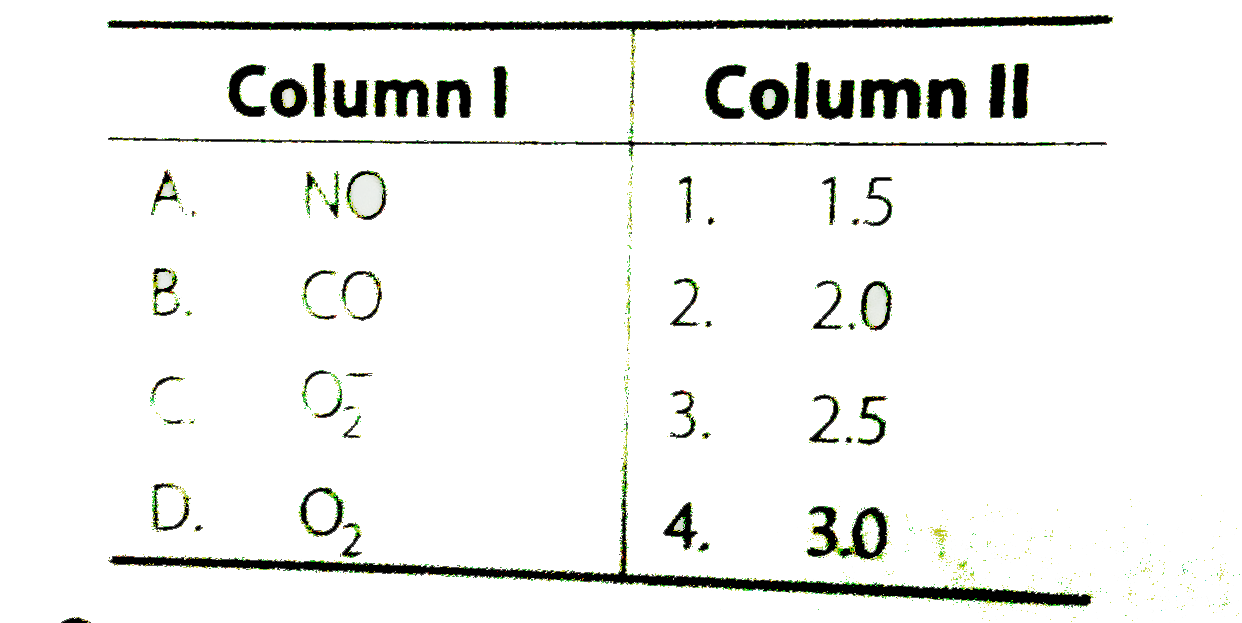Match the species in Column I with the geometry/shape in Column II.
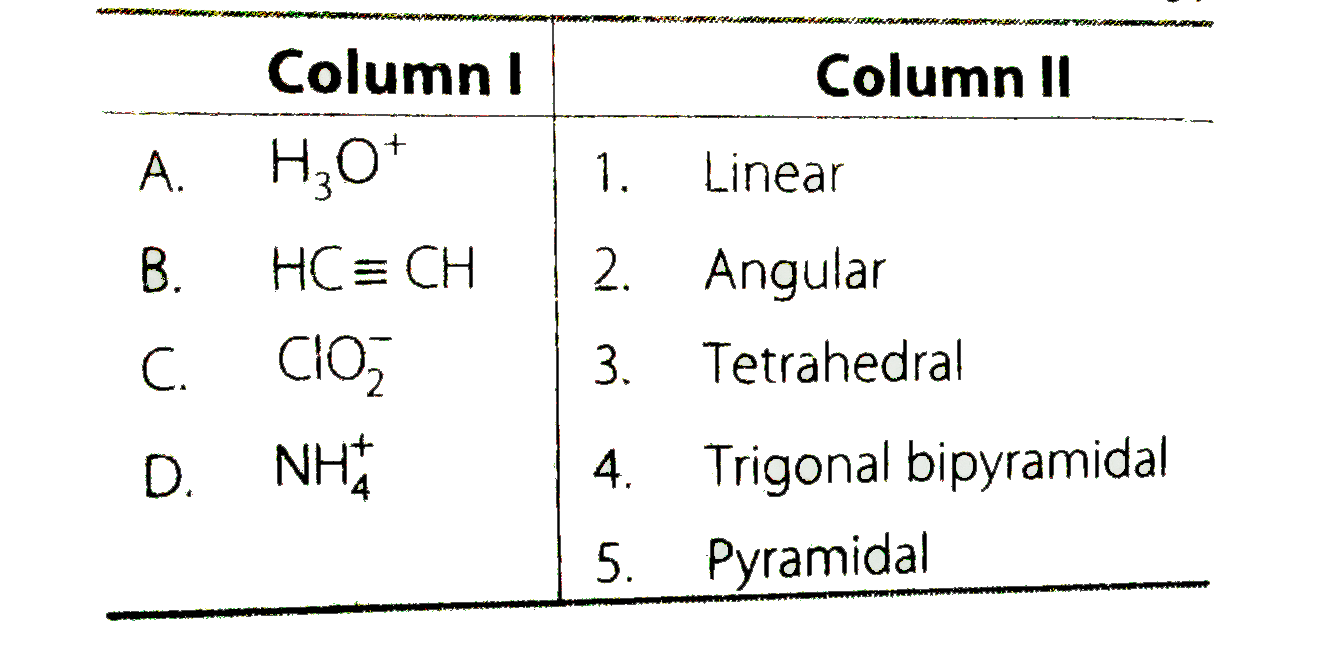
Video Solution
Text Solution
(A-5), (B-1), (C-2), (D-3)
A. H3O+ = 3bp +1p pyramidal shape.
B) HC≡CH⇒ linear as sp hybridised shape.
C) CIO−2=2bp+2Ip⇒ angular shape.
D) NH+4=4bp+0/p⇒ tetrahedral shape.
|
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:NCERT EXEMPLARChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:Assertions and Reasons
Explore 3 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:NCERT EXEMPLARChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:Long Answer type questions
Explore 9 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:NCERT EXEMPLARChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:Short answer types questions
Explore 21 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES.Book:NCERT EXEMPLARChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES.Exercise:Long answer types question
Explore 7 Videos
Similar Questions
Knowledge Check
Match the species given in Column I with the shape given in Column II and mark the correct option.
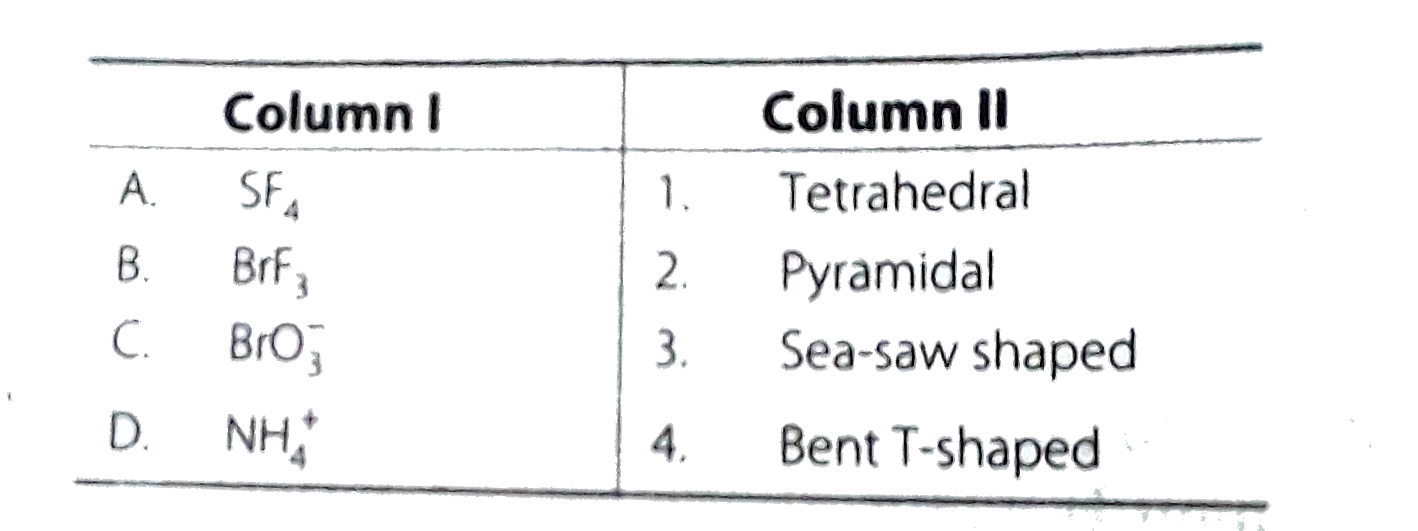 AABCD3214BABCD3421CABCD1234DABCD1432
AABCD3214BABCD3421CABCD1234DABCD1432Match the species in Column I with the bond order in Column II.
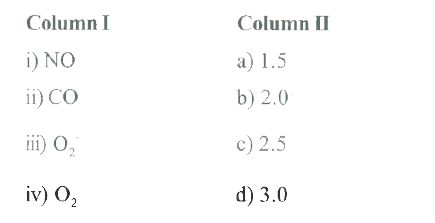 Ai-c,ii-d,iii-a,iv-bBi-a,ii-b,iii-c,iv-dCi-a,ii-b,iii-d,iv-cDi-a,ii-c,iii-e,iv-d
Ai-c,ii-d,iii-a,iv-bBi-a,ii-b,iii-c,iv-dCi-a,ii-b,iii-d,iv-cDi-a,ii-c,iii-e,iv-dMatch the species given in Column I with the shape given in Column II and mark the correct option.
 AA(3)B(2)C(1)D(4)BA(3)B(4)C(2)D(1)CA(1)B(2)C(3)D(4)DA(1)B(4)C(3)D(2)
AA(3)B(2)C(1)D(4)BA(3)B(4)C(2)D(1)CA(1)B(2)C(3)D(4)DA(1)B(4)C(3)D(2)
Match the molecule in Column I with the shape in Column II

View SolutionMatch the species in Column I with bond order in Column II.
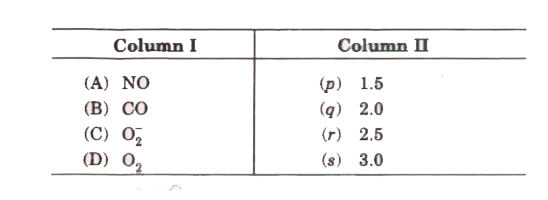
View SolutionMatch the species given in column I with prperties given in Column II.

View SolutionMatch the species given column I with prooperties given in column II

View SolutionMatch the species in Column I with the type of hybrid orbitals in Column II.
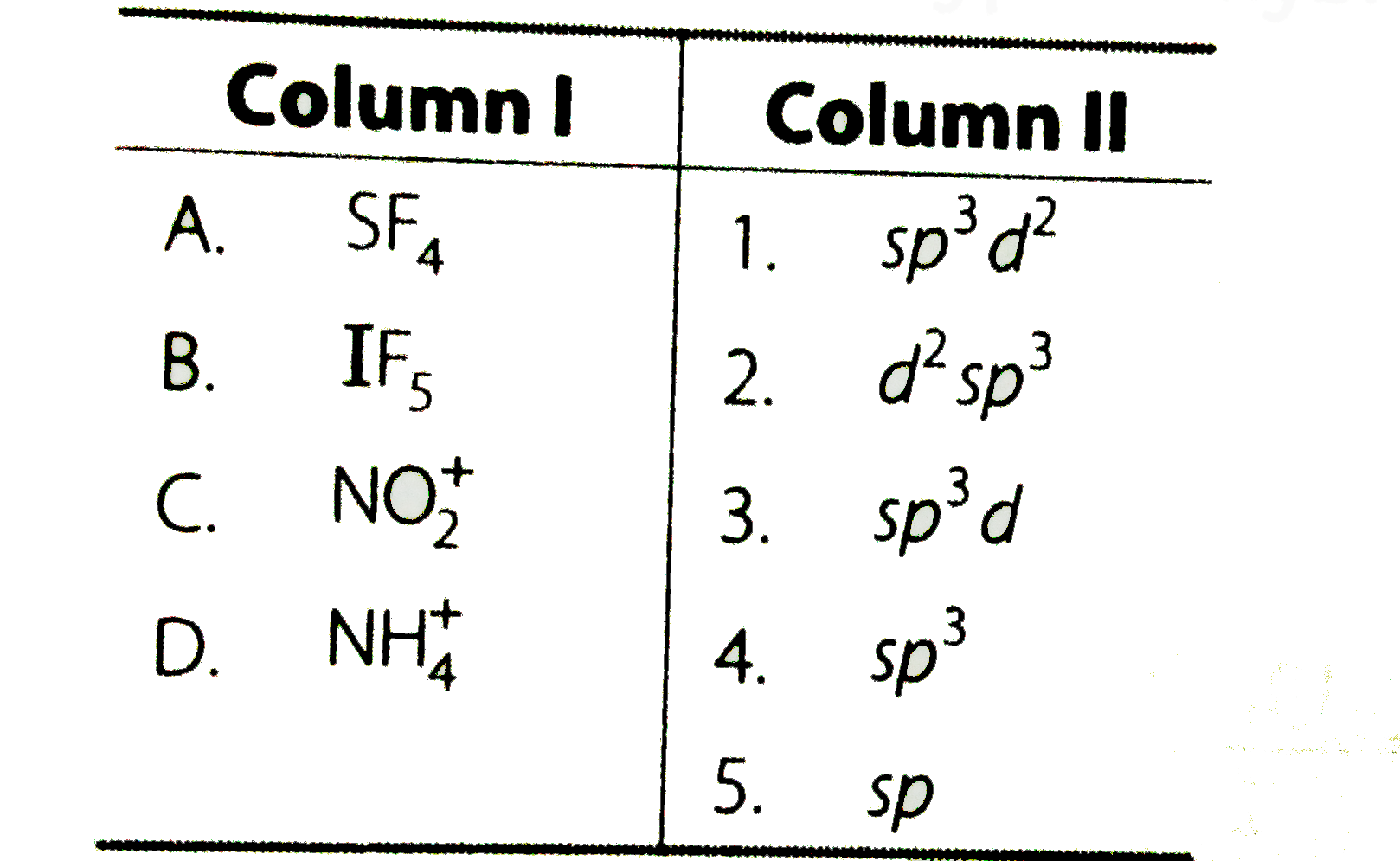
View Solution