Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond. F2, a single bond and Ne2 , no bond.
Formation of N2 molecules.
Electrons configuration of N-atom .7N=1s2,2s2,2p1x,2p1y,2p1z,N2 molecules =σ1s2,σ⋆1s2,σ2s2,π2p2x=π2p2y,π2p2z

Bond order =12[Nb−Na]=12(10−4)=3
Bond order value of 3 means that N2 contains a triple bond.
Formation of F2 molecule, .9F=1s2,2s2,2p2x,σ⋆2s2,σ2p2z,π2p2x=π2p2y,π⋆2p2z=π⋆2p2y
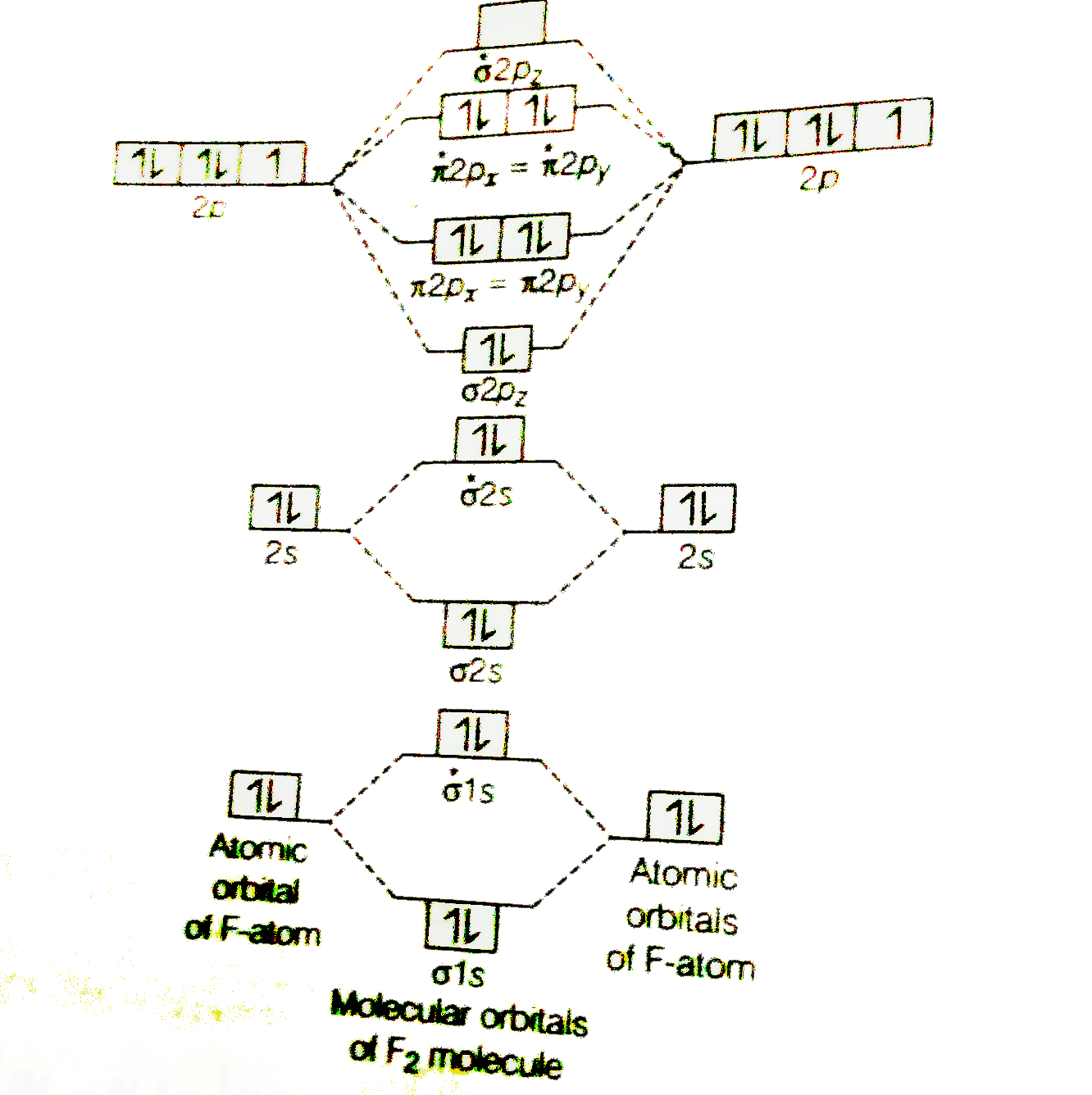
Bond order =12[Nb−Na]=12(10−8)=1
Bond order value 1 means that F2 contains single bond.
Formation of Ne2 molecule 10Ne=1s2,2s2,2p2x,2p2y,2p2z
Ne2 molecule = σ1s2,σ⋆1s2,σ2s2,σ⋆2s2,σ2p2z,π2p2x=π2p2y,π⋆2p2x=π⋆2p2y,σ⋆2p2z
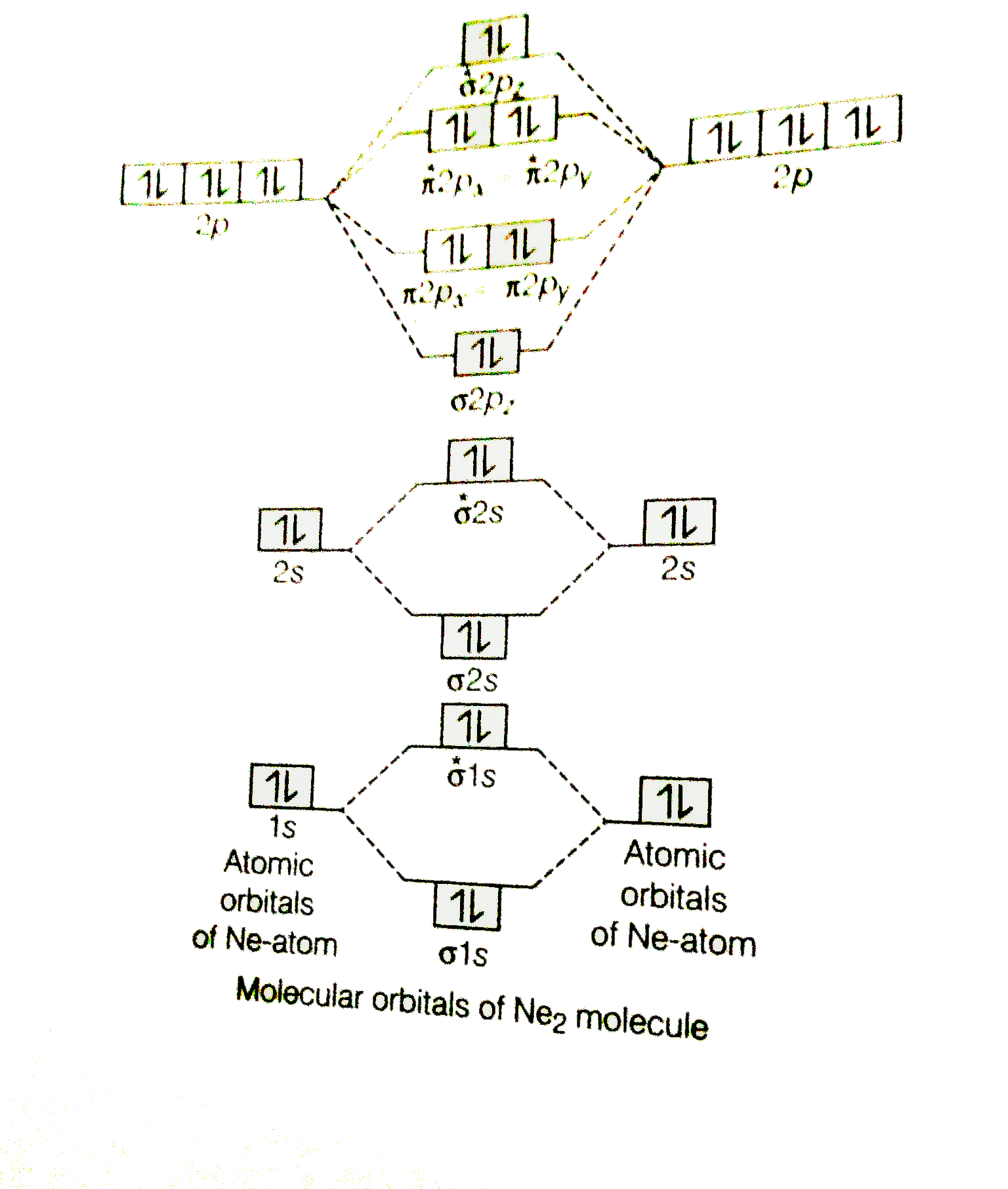
Bond order =12[Nb−Na]=12(10−10)=0
Bond order value zero means that there is no formation of bond between two Ne-atoms. Hence, Ne2 molecule does not exist.
Similar Questions
In which of the following cases C-C bond length will be highest? (I) In N_(2)F_(4) , d-orbitals are contracted by electronegative flourine atoms, but d-orbitals contraction is not possible by H-atoms in N_(2)H_(4) (II) The N-N bond energy in N_(2)F_(4) is more than N-N bond energy in N_(2)H_(4) (III) The N-N bond length in N_(2)F_(4) is more than that of in N_(2)F_(4) (IV) The N-N bond length in N_(2)F_(4) is less than that of in N_(2)H_(4) choose the correct codes:
View SolutionThe number of bonding electron pairs in N2 on the basis of molecular orbital theory is
View SolutionThe bond dissociation energy ( E) and bond length ( R) of O_(2),N_(2) and F_(2) follow the order as :
View SolutionIn O_(2) has double bond, F_(2) has single bond and Ne_(2) has no bond.
View SolutionWith the help of molecular orbital theory, show that N_(2) molecule has triple bond, O_(2) molecule has double bond while F_(2) molecule has single bond. Compare their bond strengths.
View SolutionUse the molecular orbital energy level diagram to show that N_(2) would be expected to have a triple bond. F_(2) , a single bond and Ne_(2) , no bond.
View SolutionUse the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2 a single bond and Ne2, no bond.
View Solutionआण्विक कक्षकों के ऊर्जा - स्तर आरेख द्वारा दर्शाइए कि N2 में त्रिबंध, F2 में एकल बंध तथा Ne2 में कोई बंध अपेक्षित नहीं है |
View Solution
