The concentration of R in the reaction R→P was measured as a function of time and the following data is obtained:
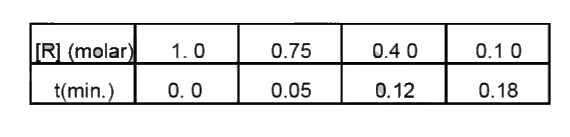
The order of the reaction is
Step by step video & image solution for The concentration of R in the reaction Rrarr P was measured as a function of time and the following data is obtained: The order of the reaction is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- Chemical KineticsBOOK - MOTIONCHAPTER - Chemical KineticsEXERCISE - Exercise - 4 (Level - II) (SUBJECTIVE PROBLEM)
1 Video
- Chemical KineticsBOOK - MOTIONCHAPTER - Chemical KineticsEXERCISE - Exercise - 4 (Level - I)
18 Videos
- CHEMICAL EQUILIBRIUMBOOK - MOTIONCHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - Exercise - 4
20 Videos
- CLASSROOM PROBLEMS BOOK - MOTIONCHAPTER - CLASSROOM PROBLEMS EXERCISE - Electrochemistry
22 Videos
Similar Questions
The concentration of R in the reaction R→P was measured as a funcation of time and the following data is obtained:
View SolutionThe concentration of R in the reaction R→P was measured as a function of time and the following data were obtained. What is the order of the reaction?
∣∣∣[R](mol)1.00.750.400.10T(min)0.00.050.120.18∣∣∣
View Solution
Knowledge Check
The concentration of R in the reaction R→P was measured as a function of time and the following data was obtained
[R]10.50.250.125Time00.050.10.15
The order of reaction is :A2B3C1DzeroFor a creactionj, the following data were obtained:
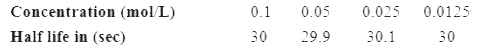
the order of reaction is:A2B1C0DfractionalFor a gaseous reaction, the following data were recorded :
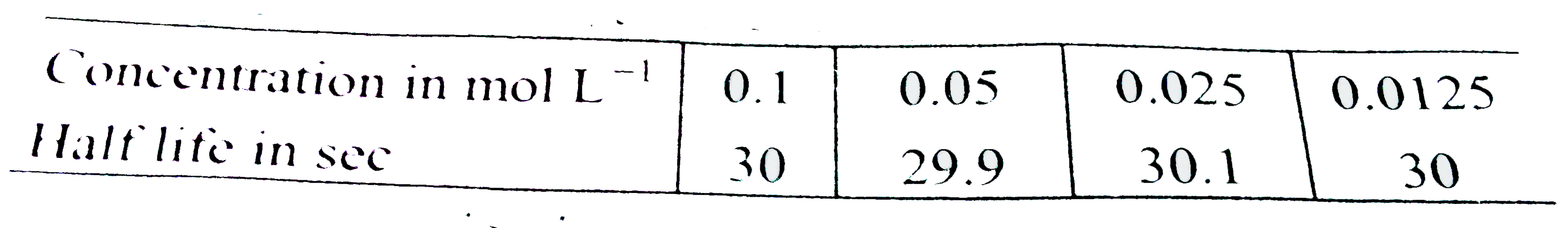
The order of reaction is :AsecondBfirstCzeroDfractional
The concentration R in the reaction R→P was measured as a function of time and the following data is obtained.

The order of reaction is
View SolutionThe concentration of "R" in the reaction R→P was measured as a function of time and the following data is obtained [[R]1098.17.29t( minutes )0.0102030]
Order of the reaction is
View SolutionAn irreversible reaction A+B→P is studied under conditions where initial concentrations of A and B are equal.Under these circumstances a graph of In A as a function of time is linear.What is the order of the reaction with respect to A with given data?
View SolutionFor a reaction between A and B, the initial rate of reaction is measured for various initial concentrations of A and B . The data provided are :
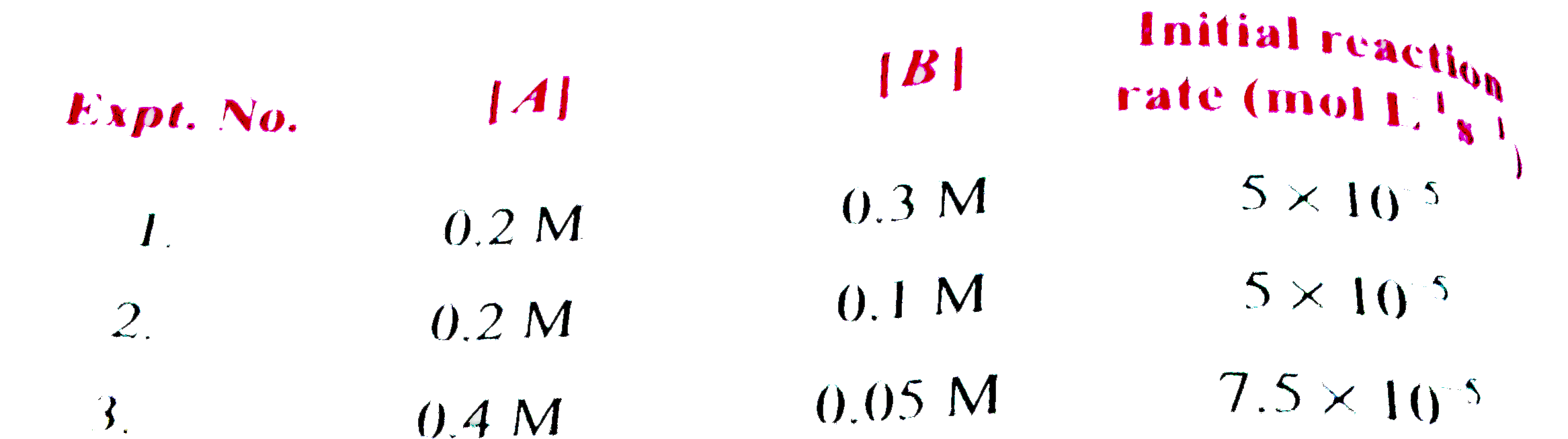
The overall order of the reaction is :
View SolutionFor a reaction between A and B, the initial rate of reaction is measured for various initial concentrations of A and B. The data provided are

The overall order of the reaction is
View Solution

