The conversion CH3CH2CHO––––→CH3CH2CH2OH can not be affected by:
NaBH4
Zn / HCl / Hg
H2/Ni
Na + alcohol
The correct Answer is:B
To solve the question regarding the conversion of propanal (CH₃CH₂CHO) to propanol (CH₃CH₂CH₂OH), we need to identify which reagent does not facilitate this conversion. The conversion involves the reduction of an aldehyde to an alcohol.
1. Identify the Reactants and Products:
- The starting compound is propanal (CH₃CH₂CHO), which is an aldehyde.
- The product is propanol (CH₃CH₂CH₂OH), which is a primary alcohol.
2. Understand the Reaction Type:
- The reaction is a reduction process where the aldehyde is converted into an alcohol.
- Aldehydes can be reduced to alcohols using various reducing agents.
3. List Possible Reducing Agents:
- Common reducing agents include:
- Lithium aluminium hydride (LiAlH₄)
- Sodium borohydride (NaBH₄)
- Hydrogen gas (H₂) in the presence of a catalyst like nickel (Ni)
- Sodium metal in alcohol (Na + alcohol)
4. Evaluate Each Option:
- Option 1: Sodium Borohydride (NaBH₄): This is a reducing agent that can convert aldehydes to alcohols. Thus, it affects the conversion.
- Option 2: Zinc Amalgam in Presence of HCl: This is used in Clemensen reduction, which converts carbonyl compounds (like aldehydes) to alkanes by removing the oxygen. Therefore, it does not facilitate the conversion to alcohol.
- Option 3: Nickel in Presence of Hydrogen: This is a reducing agent that can reduce aldehydes to alcohols. Thus, it affects the conversion.
- Option 4: Sodium + Alcohol: This also acts as a reducing agent and can facilitate the conversion of aldehydes to alcohols.
5. Conclusion:
- The reagent that does not affect the conversion of propanal to propanol is Zinc Amalgam in Presence of HCl (Option 2).
Final Answer:
The conversion CH₃CH₂CHO→CH₃CH₂CH₂OH cannot be affected by Zinc Amalgam in Presence of HCl.
---
- The starting compound is propanal (CH₃CH₂CHO), which is an aldehyde.
- The product is propanol (CH₃CH₂CH₂OH), which is a primary alcohol.
- The reaction is a reduction process where the aldehyde is converted into an alcohol.
- Aldehydes can be reduced to alcohols using various reducing agents.
- Common reducing agents include:
- Lithium aluminium hydride (LiAlH₄)
- Sodium borohydride (NaBH₄)
- Hydrogen gas (H₂) in the presence of a catalyst like nickel (Ni)
- Sodium metal in alcohol (Na + alcohol)
- Option 1: Sodium Borohydride (NaBH₄): This is a reducing agent that can convert aldehydes to alcohols. Thus, it affects the conversion.
- Option 2: Zinc Amalgam in Presence of HCl: This is used in Clemensen reduction, which converts carbonyl compounds (like aldehydes) to alkanes by removing the oxygen. Therefore, it does not facilitate the conversion to alcohol.
- Option 3: Nickel in Presence of Hydrogen: This is a reducing agent that can reduce aldehydes to alcohols. Thus, it affects the conversion.
- Option 4: Sodium + Alcohol: This also acts as a reducing agent and can facilitate the conversion of aldehydes to alcohols.
- The reagent that does not affect the conversion of propanal to propanol is Zinc Amalgam in Presence of HCl (Option 2).
The conversion CH₃CH₂CHO→CH₃CH₂CH₂OH cannot be affected by Zinc Amalgam in Presence of HCl.
Similar Questions
Knowledge Check
The conversion,
CH3CH=CHCHO→CH3CH=CHCH2OH, can be affected with :AH2/NiBZn/HClCNaBH4Dnone of theseCH3−CH2−CHO&CH2=CH−CH2OH are
AFunctionBTaut omersCPositionDMetamers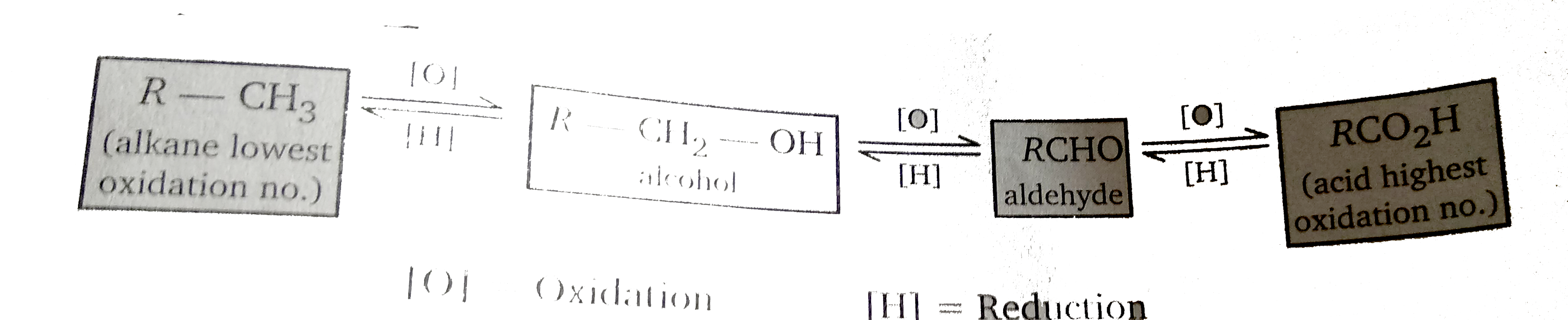
Conversion (CH3−CH3→CH3−CH2−OH) alkane → alcohol is achieved by :ABr2/hv, alc. KOHBBr2/hv, aq. KOHCBr2/CCl4,LiAlH4DBr2/CCl4,NaBH4
How would you carry out the following conversions ?
(a) H3CCH2=CH2→H3C−CH2CH2CH2OH
(b) H3CCH2−CH=CH2→H3C−CH2−CH(OH)CH2OH
(c) H3CC≡CH→H3CCOCH3
(d) Br2CHCHBr2→HC≡CH
View SolutionCH3COCH3andCH3CH2CHO can be distinguished by :
View SolutionThe reaction ,
CH3CHO+H2N−NH2→CH3=N.NH2 is :
View SolutionThe reaction
CH3CHO+CH3CHODil. alkali−−−−−→CH3CH(OH)CH2CHO is known as
View SolutionCH3CHOOH−−−→CH3CH(OH)CH2CHO represents
View Solution

