Cyanohydrin of which compound on hydrolysis will give lactic acid?
C6H5CHO
HCHO
CH3CHO
CH3−CH2−CHO
The correct Answer is:C
To determine which compound's cyanohydrin will yield lactic acid upon hydrolysis, we need to analyze the structure of lactic acid and the possible cyanohydrins formed from the given compounds.
1. Identify the Structure of Lactic Acid:
- Lactic acid has the chemical formula C3H6O3 or can be represented as CH3CH(OH)COOH.
- This indicates that lactic acid contains a hydroxyl group (-OH) and a carboxylic acid group (-COOH).
2. Consider the Cyanohydrin Formation:
- Cyanohydrins are formed from carbonyl compounds (aldehydes or ketones) when they react with hydrogen cyanide (HCN).
- The reaction involves the nucleophilic attack of the cyanide ion (CN⁻) on the carbonyl carbon, leading to the formation of a cyanohydrin.
3. Evaluate Each Compound:
- Option 1: Benzaldehyde (C₆H₅CHO):
- Upon reaction with HCN, it forms C6H5CH(OH)CN.
- Hydrolysis of this cyanohydrin yields C6H5CH(OH)COOH, which is not lactic acid (it has a phenyl group).
- Option 2: Formaldehyde (HCHO):
- Reaction with HCN gives H2C(OH)CN.
- Hydrolysis leads to H2C(OH)COOH, which is not lactic acid.
- Option 3: Acetaldehyde (CH₃CHO):
- Reaction with HCN results in CH3CH(OH)CN.
- Hydrolysis converts the cyanide to a carboxylic acid, yielding CH3CH(OH)COOH, which is indeed lactic acid.
- Option 4: Propionaldehyde (CH₃CH₂CHO):
- Reaction with HCN gives CH3CH2CH(OH)CN.
- Hydrolysis results in CH3CH2CH(OH)COOH, which is not lactic acid.
4. Conclusion:
- The only compound that yields lactic acid upon hydrolysis of its cyanohydrin is Acetaldehyde (CH₃CHO).
- Therefore, the correct answer is Option 3: CH₃CHO.
- Lactic acid has the chemical formula C3H6O3 or can be represented as CH3CH(OH)COOH.
- This indicates that lactic acid contains a hydroxyl group (-OH) and a carboxylic acid group (-COOH).
- Cyanohydrins are formed from carbonyl compounds (aldehydes or ketones) when they react with hydrogen cyanide (HCN).
- The reaction involves the nucleophilic attack of the cyanide ion (CN⁻) on the carbonyl carbon, leading to the formation of a cyanohydrin.
- Option 1: Benzaldehyde (C₆H₅CHO):
- Upon reaction with HCN, it forms C6H5CH(OH)CN.
- Hydrolysis of this cyanohydrin yields C6H5CH(OH)COOH, which is not lactic acid (it has a phenyl group).
- Option 2: Formaldehyde (HCHO):
- Reaction with HCN gives H2C(OH)CN.
- Hydrolysis leads to H2C(OH)COOH, which is not lactic acid.
- Reaction with HCN results in CH3CH(OH)CN.
- Hydrolysis converts the cyanide to a carboxylic acid, yielding CH3CH(OH)COOH, which is indeed lactic acid.
- Reaction with HCN gives CH3CH2CH(OH)CN.
- Hydrolysis results in CH3CH2CH(OH)COOH, which is not lactic acid.
- The only compound that yields lactic acid upon hydrolysis of its cyanohydrin is Acetaldehyde (CH₃CHO).
- Therefore, the correct answer is Option 3: CH₃CHO.
Similar Questions
Knowledge Check
The cyanohydrin of a carbonyl compound on hydyrolysis gives lactic acid. The carbonyl compound is
AHCHOBCH3CHOCCH3COCH3DCH3COCH2CH3The following compound on hydrolysis will give
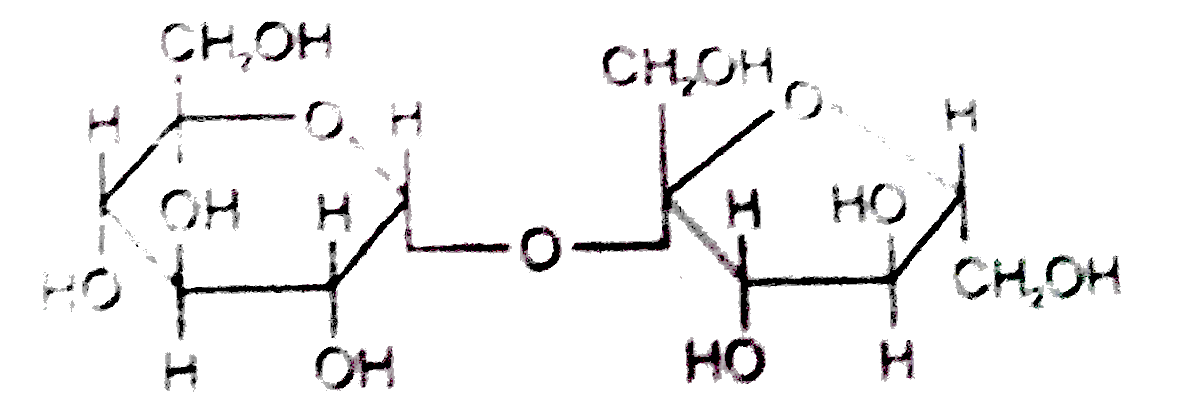 AA pair of anomersBA pair of enantiomerisCA pair of epimersDA pair of molecules having common tautomer
AA pair of anomersBA pair of enantiomerisCA pair of epimersDA pair of molecules having common tautomerCyanohydrine of the following compound on hydrolysis give optically active compound.
ACH3−COCH3BH−CHOCCH3−CHODAll of these
Compound 'A' undergoes formation of cyanohydrin which on hydrolysis gives lactic acid [CH3CH(OH)COOH] Therefore, compound 'A' is :
View SolutionCompound X undergoes formation of cyanohydrin which on hydrolysis gives lactic acid (CH3CH(OH)COOH). Therefore, compound X is
View SolutionA cyanohyrdrin of a compound (X) on hydrolysis gives an α-hydroxy acid which shows optical activity after resolution . The compound (X) is:
View SolutionWhich of the following compounds on hydrolysis yields acetic acid?
View SolutionA carbonyl compound 'A'' react with hydrogen cyanide to give cyanohydrin 'B' which on acid hydrolysis gives optically active α - hydroxy propanoic acid 'C' Compounds A, B, C respectively are ,
View Solution

