Whcih of following give positive test with 2,4-DNP?
CH3−O∣∣C−H3
CH3−O∣∣C−CH3
CH3−O∣∣C−OH
CH3−CH2−OH
The correct Answer is:A, B
To determine which compounds give a positive test with 2,4-Dinitrophenylhydrazine (2,4-DNP), we need to follow these steps:
1. Understand the Reagent:
- 2,4-DNP stands for 2,4-Dinitrophenylhydrazine, which is a reagent used to test for the presence of carbonyl groups (C=O) in aldehydes and ketones.
2. Identify Carbonyl Compounds:
- The compounds that will give a positive test with 2,4-DNP are those that contain carbonyl groups, such as aldehydes and ketones.
3. Analyze the Given Compounds:
- Let's consider the options provided (though they are not explicitly listed here, we will assume there are some common organic compounds).
- For example, if the options include:
- A: Acetone (CH3COCH3)
- B: Butanal (CH3(CH2)2CHO)
- C: Ethanol (CH3CH2OH)
- D: Acetic acid (CH3COOH)
4. Determine Which Compounds React:
- Acetone (A): This is a ketone and will react with 2,4-DNP to form a yellow/orange precipitate.
- Butanal (B): This is an aldehyde and will also react with 2,4-DNP to form a yellow/orange precipitate.
- Ethanol (C): This is an alcohol and does not contain a carbonyl group, hence it will not react with 2,4-DNP.
- Acetic acid (D): This is a carboxylic acid and also does not contain a carbonyl group in the form required for the 2,4-DNP reaction, hence it will not react.
5. Conclusion:
- The compounds that give a positive test with 2,4-DNP are Acetone (A) and Butanal (B).
Final Answer:
- The correct options that give a positive test with 2,4-DNP are Acetone (A) and Butanal (B).
- 2,4-DNP stands for 2,4-Dinitrophenylhydrazine, which is a reagent used to test for the presence of carbonyl groups (C=O) in aldehydes and ketones.
- The compounds that will give a positive test with 2,4-DNP are those that contain carbonyl groups, such as aldehydes and ketones.
- Let's consider the options provided (though they are not explicitly listed here, we will assume there are some common organic compounds).
- For example, if the options include:
- A: Acetone (CH3COCH3)
- B: Butanal (CH3(CH2)2CHO)
- C: Ethanol (CH3CH2OH)
- D: Acetic acid (CH3COOH)
- Acetone (A): This is a ketone and will react with 2,4-DNP to form a yellow/orange precipitate.
- Butanal (B): This is an aldehyde and will also react with 2,4-DNP to form a yellow/orange precipitate.
- Ethanol (C): This is an alcohol and does not contain a carbonyl group, hence it will not react with 2,4-DNP.
- Acetic acid (D): This is a carboxylic acid and also does not contain a carbonyl group in the form required for the 2,4-DNP reaction, hence it will not react.
- The compounds that give a positive test with 2,4-DNP are Acetone (A) and Butanal (B).
- The correct options that give a positive test with 2,4-DNP are Acetone (A) and Butanal (B).
Similar Questions
Knowledge Check
Glucose gives positive test with
AFehling's solutionBTollens' reagentCSchiff's reagentDSodium bisulphite.Which of following give positive Fehling solution test
ASucroseBGlucoseCFatsDProteinWhich of following gives positive carbylamine test ?
A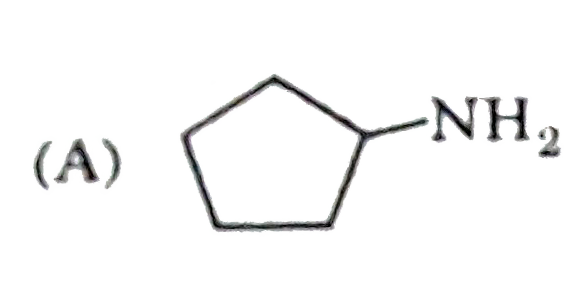 B
B C
C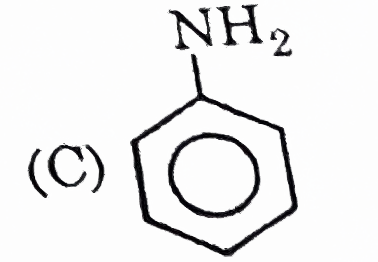 D
D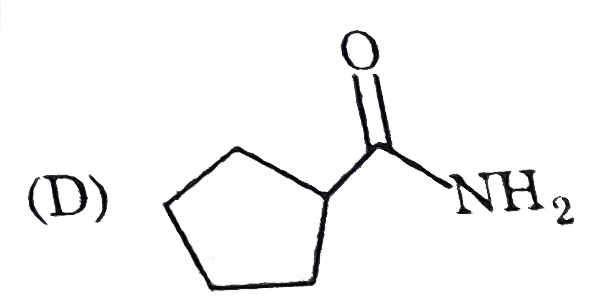
Which of the following will give positive tollen's test ?
View SolutionCompound 'X' give positive test with 2,4- DNP and with I2/NaOH compound (X) may be :
View SolutionCompound 'X' give positive test with 2,4- DNP and with I2/NaOH compound (X) may be :
View SolutionObserve the following sequence of reactions

P shows geometrical isomers.Q gives positive Tollen's test and the oxidation product of Tollen's test followed by acidification is the strongest acis among its all position isomers.
R gives positive lab test with 2,4,-DNP, Fehling solutioni and I2/NaOH reagents.
What could be the structure of Q?
[GOC−POC]
View SolutionObserve the following sequence of reations
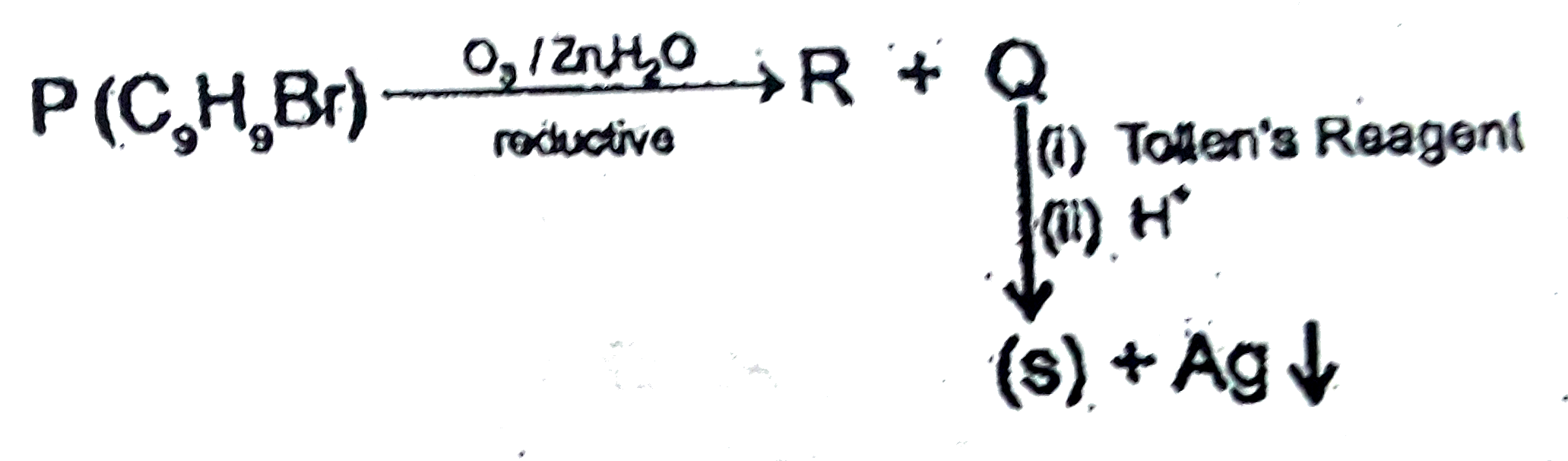
P shows geometrical isomersm.Q gives positive Tollen's test and the oxidation product of Tollen's test followed by acidification is the strongest acid among its all position isomers. R gives positive lab tests with 2,4-DNP, Fehling solution and I2/NaOH reagents.
Identify the structure of R.
View Solution

