Pressure over ideal binary liquid mixture containing 10 moles each of liquid A and B is gradually decreased isothermally. If P∘A = 200 mm Hg and P∘B = 100 mm Hg, find the pressure at which half of the liquid is converted into vapour.
The correct Answer is:0.04
Similar Questions
Two liquids A and B have vapour pressure of 500 mm Hg and 200 mm Hg respectively. Calculate the mole fraction of A at which two liquids have equal partial pressures.
View SolutionTwo liquids A and B form an ideal solution. At 300 K, the vapour pressure of a solution containing 1 mole of A and 3 moles of B is 550 mm of Hg. At the same temperature, if one mole of B is added to this solution, the vapour pressure of the solution increases by 10 mm of Hg. Determine the vapour pressure of A and B in their pure states
View Solution
Knowledge Check
A mixture contains 1 mole of volatile liquid A (P0A=80mm Hg). and 3 moles of volatile liquid B (P0A=80mm Hg). If solution behaves ideally, the total vapour pressure of the distillate is
A25 mm HgB85 mm HgC90 mm HgD92 mm HgAt 300 K the vapour pressure of an ideal solution containing 1 mole of liquid A and 2 moles of liquid B is 500 mm of Hg. The vapour pressure of the solution increases by 25 mm of Hg, if one more mole of B is added to the above ideal solution at 300K. Then the vapour pressure of A in its pure state is
A300 mm of HgB400 mm of HgC500 mm of HgD600 mm HgIf same amount of gas is trapped over liquid (a) and liquid (b) in following containers. Assuming temperature and cross-sectional area of container are same. Patm=760mmHg,dHg=13.6g/mL
Find the pressure (in cm Hg) of gas over the liquid (b).
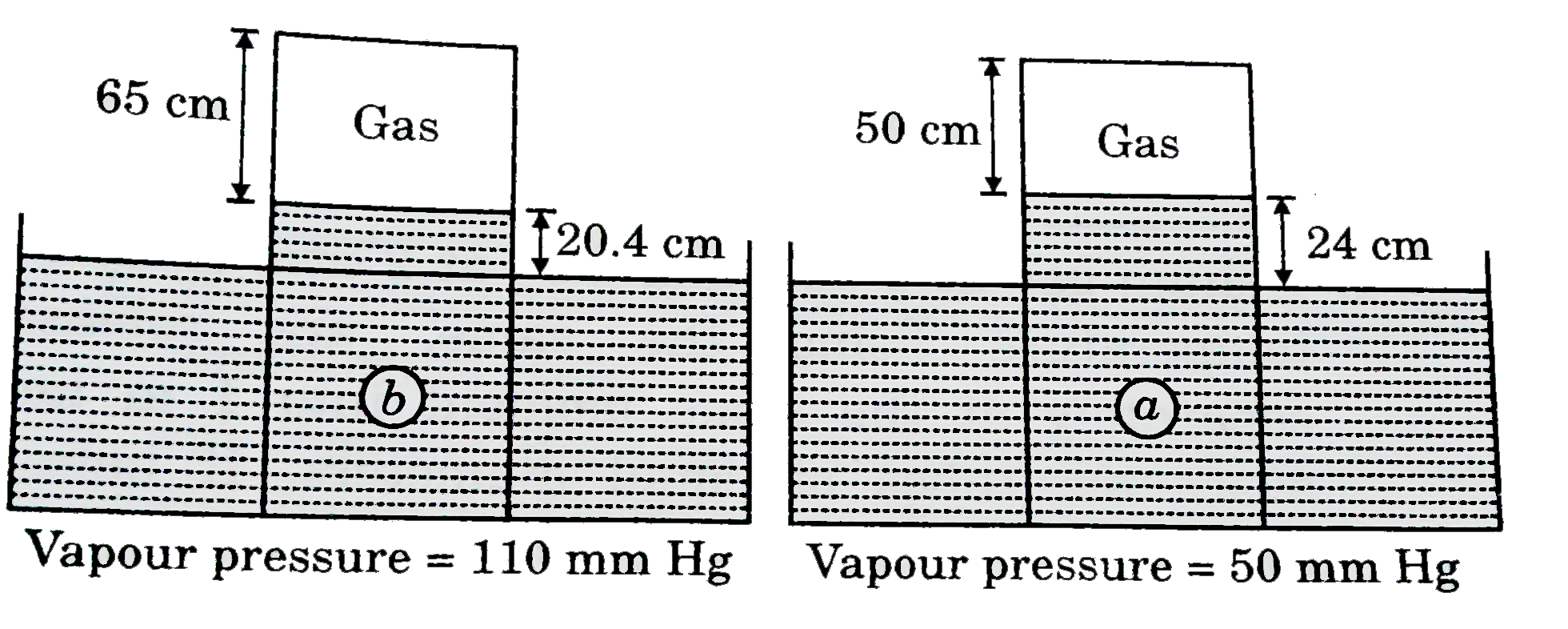 A76 cmB11 cmC50 cmD65 cm
A76 cmB11 cmC50 cmD65 cm
The vapour pressure of pure liquid A and liquid B at 350 K are
440 mm and 720 mm of Hg. If total vapour pressure of solution is
580 mm of Hg then the mole fraction of liquid A in vapour
phase will be :-
View SolutionThe total vapour pressure of a mixture of 1 mole of A (PA=200mmHg) and 3 mole of B (PB=360 mm Hg) is 350 mm Hg. Then
View SolutionA mixture contains 1 mole of volatile liquid A (P0A=80mm Hg). and 3 moles of volatile liquid B (P0A=80mm Hg). If solution behaves ideally, the total vapour pressure of the distillate is
View SolutionThe total vapour pressure of a mixture of 1 mole of A (PA=200mmHg) and 3 mole of B (PB=360 mm Hg) is 350 mm Hg. Then
View SolutionThe vapour pressure of pure liquid A and liquid B at 350 K are 440 mm and 720 mm of Hg. If total vapour [ressure of solution is 580 mm of Hg then the mole fraction of liquid A in vapour phase will be :-
View Solution

