The rate of a reaction A + B → products is studied to give following data.
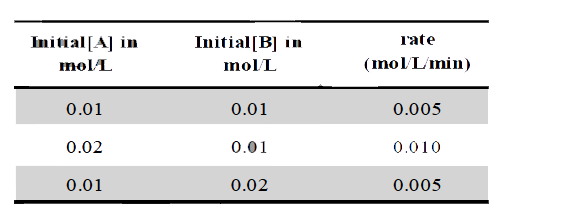
What is the rate law ? What is the half-life of A in the reaction
Step by step video & image solution for The rate of a reaction A + B to products is studied to give following data. What is the rate law ? What is the half-life of A in the reaction by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
The following rate data were obtained at 303 K for the following reaction:
2A+B→C+D

What is the rate law? What is the order with respect to each rectant and the overall order? What are the units of the rate constant?
View SolutionThe rate of reaction A+B→ Products is given by Rate =k[A]1/2[B]2. What are the units of the rate constant ?
View Solution
Knowledge Check
For the reaction between A and B
A+B→ product
the following rate data were obtained .
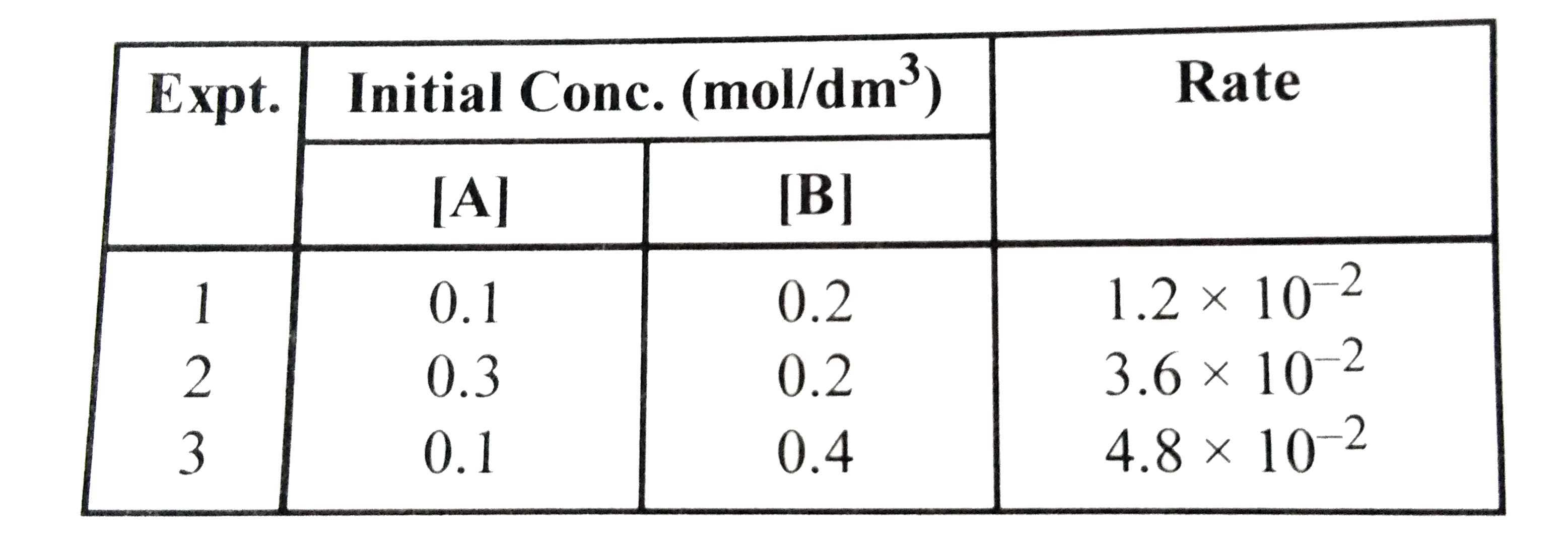
the reaction isAZero orderBfirst orderCsecond orderDthird orderFor a reaction ,A+B→C data is as follows :
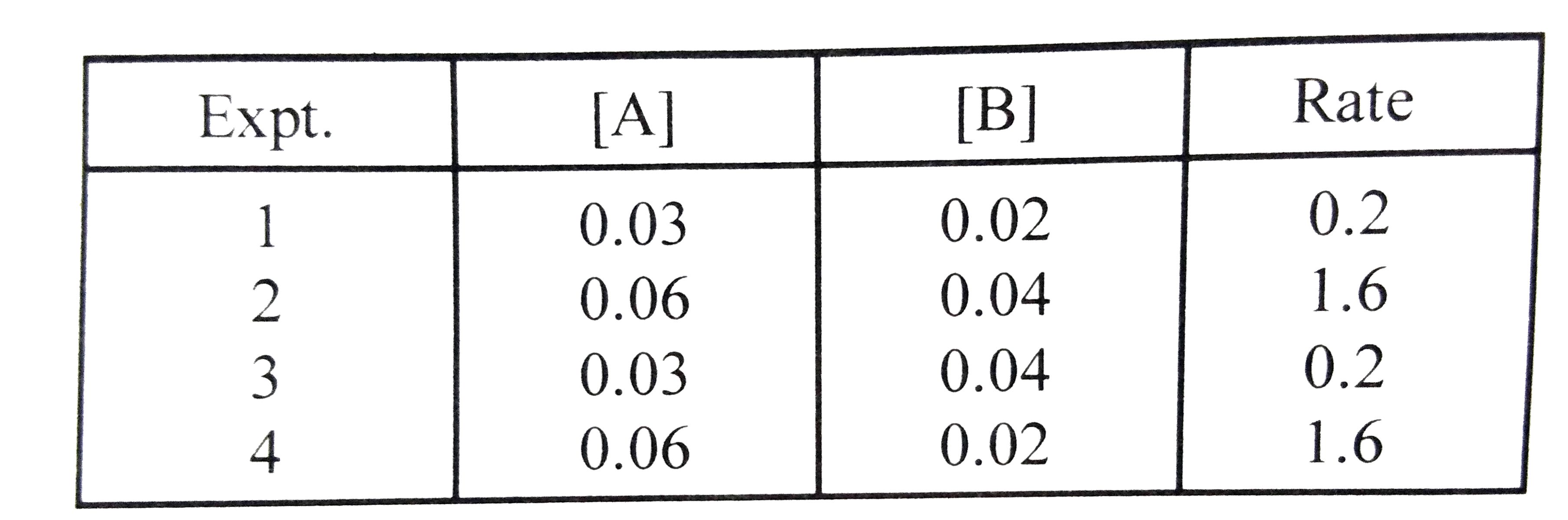
the rate law for the reaction isARate =K[B]3BRate =[A]2[B]CRate =k[A]3DRate =[A][B]2
For the reaction A+2B→ product, the reaction rate was halved as the concentration of A was doubled. What is the order of reaction with respect to A?
View SolutionRate of reaction, A+B→ products is given below as a function of different initial concentration of
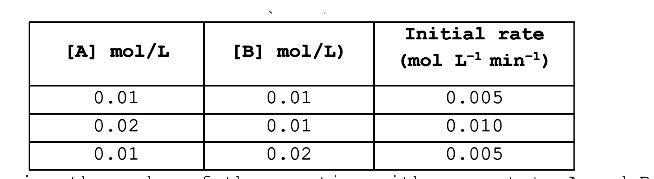
Determine the order of the reaction with respect to A and B. What is the half-life of A in the reaction?
View SolutionIf in a chemical reaction, A+B→Products, the rate law is given the expression : rate = k[A](12)[B]32. What is the order of the reaction?
View SolutionFor the given reaction
A+B→ Products,
the following data are given.
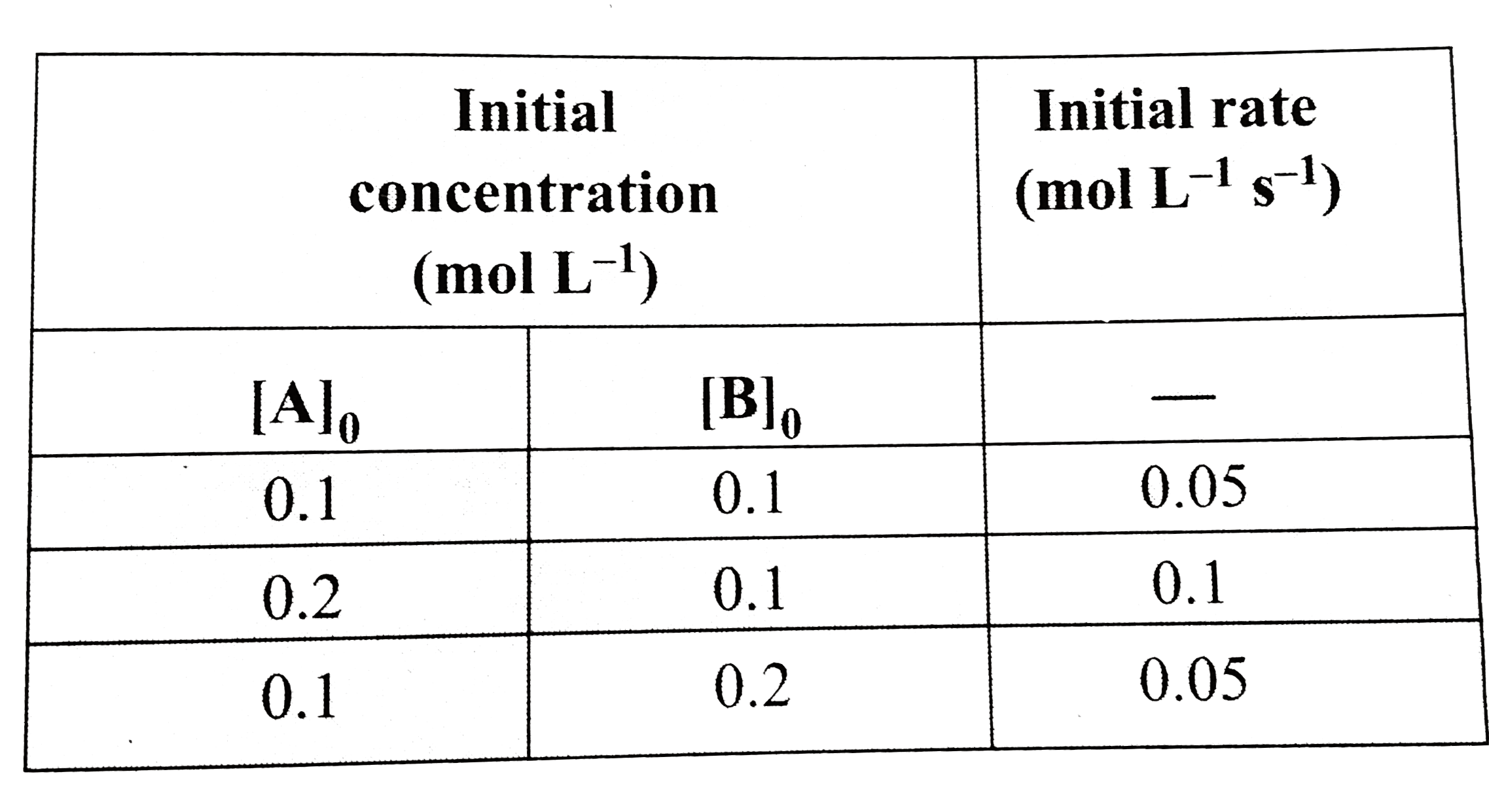
(a) Write the rate equation.
(b) Calculate the rate constant.
View SolutionWhat are the units for the rate of the reaction A→B ?
View Solutionthe rate law of the reaction A→ Product is : rate = k[A]. It has been graphically represented. What is the rate constant for the reaction?

View Solution
