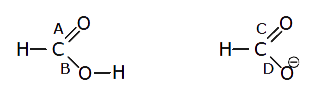
(C – O) bond lengths designated by A,B,C are in order :
A = C lt B = D
A lt B lt C = D
A lt C = D lt B
All equal
The correct Answer is:C
Step by step video, text & image solution for (C – O) bond lengths designated by A,B,C are in order : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CARBOXYLIC ACIDBook:MOTIONChapter:CARBOXYLIC ACIDExercise:Exercise - 2(LEVEL - 2)
Explore 65 Videos
- CARBOXYLIC ACIDBook:MOTIONChapter:CARBOXYLIC ACIDExercise:Exercise - 3
Explore 16 Videos
- CARBOXYLIC ACIDBook:MOTIONChapter:CARBOXYLIC ACIDExercise:Exercise - 4 (LEVEL - 2 )
Explore 22 Videos
- CARBONL COMPOUNDSBook:MOTIONChapter:CARBONL COMPOUNDSExercise:EXERCISE - 4 LEVEL -II
Explore 24 Videos
- CHEMICAL BONDING Book:MOTIONChapter:CHEMICAL BONDING Exercise:EXERCISE -4 LEVEL-II
Explore 40 Videos
Similar Questions
Knowledge Check
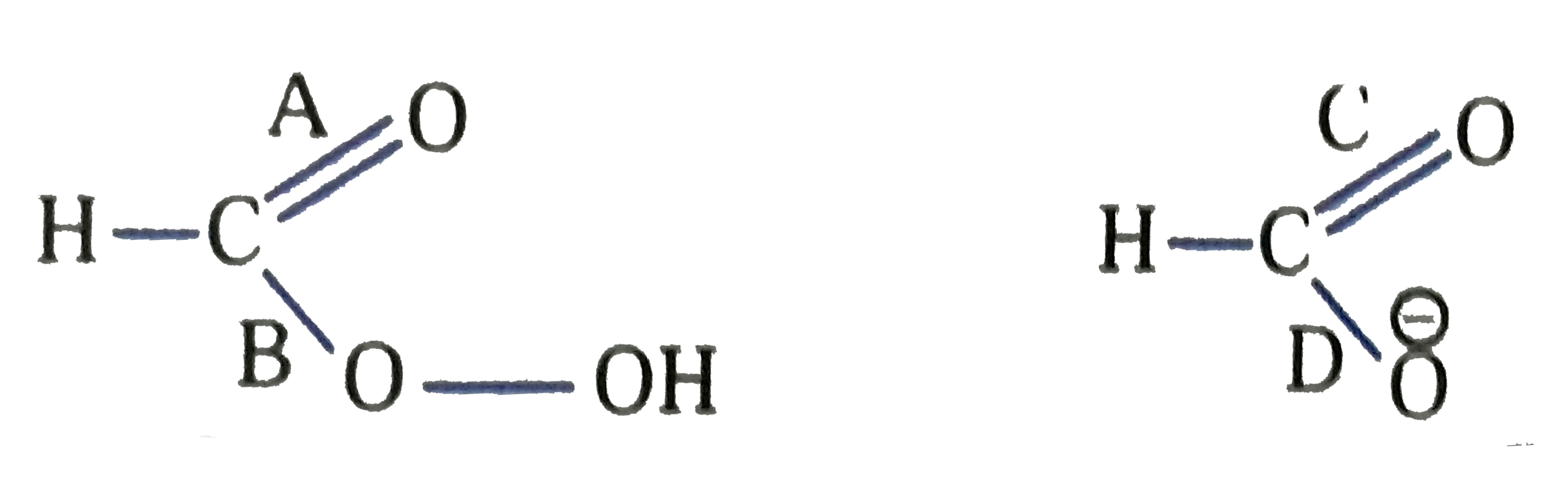
(C-O) bond lengths designated by A, B, C and D are in orderAA=C<B=DBA<B<C=DCA<C=D<BDAll equalThe bond length in C=C is
A1.20ÅB1.34ÅC1.54ÅD2.12ÅThe C-O bond length is the shortest in:
A[Cr(CO)6]B[Mo(CO)6]C[Mn(CO)6]+D[V(CO)6]−
The decreasing order of O - O bonds length in the following molecules is:
View SolutionThe decreasing order of O - O bonds length in the following molecules is:
View SolutionGiven the order of density:
Diamond>Graphite>Fullerence C60, choose thye correct order for C−C bond length (Consider large bond length if there are two different bondlengths)
View SolutionThe correct order in which the O−O bond length increases in the following :
View SolutionThe correct order in which the O-O bond length increases in the following:
View Solution