Which is/are corect statement ?
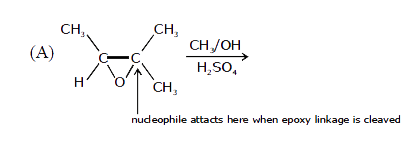
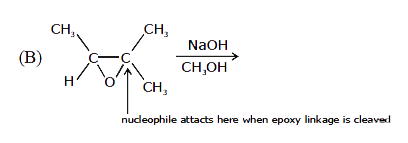
Thisisonlyaffected∈reduction→2^@ alcohol 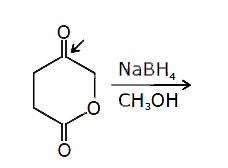
The correct Answer is:A, C, D
To determine which statements are correct regarding the reactions involving ethers and alcohols, we will analyze each statement step by step based on the information provided in the video transcript.
1. Analyze Statement A:
- The statement discusses a reaction occurring in the presence of acid, favoring the SN1 mechanism.
- In SN1 reactions, the nucleophile attacks the more substituted carbon due to carbocation stability.
- The mechanism involves the formation of a carbocation after the leaving group departs.
- Since the nucleophile attacks from the more substituted side, this statement is correct.
2. Analyze Statement B:
- This statement involves a reaction in the presence of NaOH, which is a base, favoring the SN2 mechanism.
- In SN2 reactions, the nucleophile attacks from the less hindered side to avoid steric hindrance.
- If the statement claims that the nucleophile will attack from the more hindered side, it is incorrect.
3. Analyze Statement C:
- This statement involves the reduction of a carbonyl compound using NaBH4.
- NaBH4 is a mild reducing agent that can reduce aldehydes and ketones but not esters.
- The statement claims that NaBH4 will only reduce the carbonyl carbon and not the ester, which is correct.
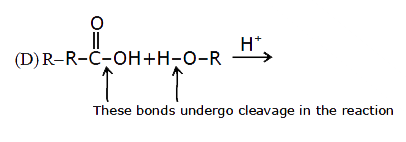
4. Analyze Statement D:
- This statement describes the reaction between an acid and an alcohol in the presence of acid (H+), leading to ester formation.
- The mechanism involves protonation of the alcohol, followed by nucleophilic attack and subsequent bond cleavage.
- The statement correctly describes the cleavage of the acid and alcohol, indicating that the OH group comes from the acid and the H from the alcohol.
- Therefore, this statement is also correct.
Conclusion:
The correct statements are A, C, and D. Statement B is incorrect.
Summary of Correct Statements:
- A: Correct (SN1 mechanism favors attack at the more substituted carbon in the presence of acid).
- B: Incorrect (SN2 mechanism favors attack at the less hindered side in the presence of a base).
- C: Correct (NaBH4 reduces carbonyls but not esters).
- D: Correct (the reaction between acid and alcohol leads to ester formation with proper bond cleavage).
- The statement discusses a reaction occurring in the presence of acid, favoring the SN1 mechanism.
- In SN1 reactions, the nucleophile attacks the more substituted carbon due to carbocation stability.
- The mechanism involves the formation of a carbocation after the leaving group departs.
- Since the nucleophile attacks from the more substituted side, this statement is correct.
- This statement involves a reaction in the presence of NaOH, which is a base, favoring the SN2 mechanism.
- In SN2 reactions, the nucleophile attacks from the less hindered side to avoid steric hindrance.
- If the statement claims that the nucleophile will attack from the more hindered side, it is incorrect.
- This statement involves the reduction of a carbonyl compound using NaBH4.
- NaBH4 is a mild reducing agent that can reduce aldehydes and ketones but not esters.
- The statement claims that NaBH4 will only reduce the carbonyl carbon and not the ester, which is correct.
- This statement describes the reaction between an acid and an alcohol in the presence of acid (H+), leading to ester formation.
- The mechanism involves protonation of the alcohol, followed by nucleophilic attack and subsequent bond cleavage.
- The statement correctly describes the cleavage of the acid and alcohol, indicating that the OH group comes from the acid and the H from the alcohol.
- Therefore, this statement is also correct.
The correct statements are A, C, and D. Statement B is incorrect.
- A: Correct (SN1 mechanism favors attack at the more substituted carbon in the presence of acid).
- B: Incorrect (SN2 mechanism favors attack at the less hindered side in the presence of a base).
- C: Correct (NaBH4 reduces carbonyls but not esters).
- D: Correct (the reaction between acid and alcohol leads to ester formation with proper bond cleavage).
Similar Questions
Knowledge Check
Which is not correct statement ?
ACasseterite, chromite and haematitie are concentrated by hydraulic washing (Tabling).BPure Al2O3 is obtained from the bauxite ore by leaching in the Bayer's processCSulphide ore is concentrated by calcination methodDRoasting can convert sulphide into oxide or dulphate and part of sulphide may also act as a reducing agentWhich of the following is/are correct statement/statements ?
AGuanidine ⎡⎢ ⎢⎣NH2−C∣∣NH−NH2⎤⎥ ⎥⎦ is more basic than pyridine because conjugate acid of guanidine has three equal contributing resonating structure.BDiethylamine is stronger base than triethylamine in aqueous mediumCOrtho-methyl aniline is weaker base than para-methyl aniline.D2,4,6-Trinitro-N,N-dimethyl aniline is stronger base than 2,4,6-Trinitro aniline.Among the following statements which is the incorrect statement?
ACaO absorbs CO2 and viontely reacts with water.BSodium amalgam reacts with hot water and gives NaOH with the liberation of H2CKO2 absorbs CO2 and releases CO.DGypsum, on heating ar 393 K, gives calcium sulphate hymiydrate (Plaster of Paris)
Which of the following is a correct statement?
View SolutionWhich one is not corect statement regarding minimizing enviromental pollution ?
View SolutionWhich of the following statements are correct statements ?
View SolutionWhich one is not a statement :
View SolutionWhich of the following statements is an incorrect statement?
View Solution
