If a molecule contains one carbon atom carrying four different groups it will not have a plane of symmetry and must therefore be chiral. A carbon atom carrying four different groups is a steregoenic or chiral centre. A structure with a plane of symmetry is achiral and superimposable on its mirror image and cannot exist as two enantiomer. A structure without a plane of symmetry is chiral and not superimposable on its mirror image and can exist as two enantiomer.
Compound has both center of symmetry and P.O.S
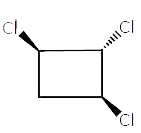
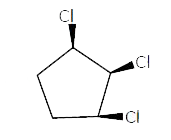
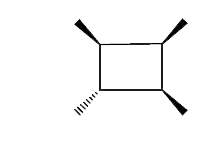
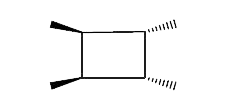
The correct Answer is:D
Similar Questions
If a molecule contains one carbon atom carrying four different groups it will not have a plane of symmetry and must therefore be chiral. A carbon atom carrying four different groups is a steregoenic or chiral centre. A structure with a plane of symmetry is achiral and superimposable on its mirror image and cannot exist as two enantiomer. A structure without a plane of symmetry is chiral and not superimposable on its mirror image and can exist as two enantiomer.
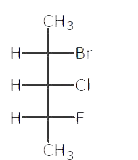
Number of enantiomeric pairs are
View SolutionIf a molecule contains one carbon atom carrying four different groups it will not have a plane of symmetry and must therefore be chiral. A carbon atom carrying four different groups is a steregoenic or chiral centre. A structure with a plane of symmetry is achiral and superimposable on its mirror image and cannot exist as two enantiomer. A structure without a plane of symmetry is chiral and not superimposable on its mirror image and can exist as two enantiomer.
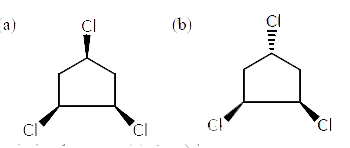
Relation between (a) & (b) is
View Solution
Knowledge Check
Number of chiral carbon atoms is
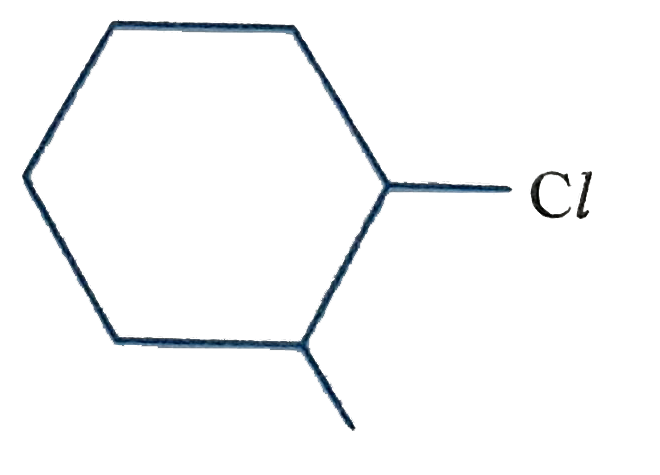 isA2B3C4D1
isA2B3C4D1A carbon atom is chiral when
Ait has tetrahdral orientationBit is sp3 hybridizedCit has four different ligandsDit forms multiple bondsAssertion: Molecules that are not superimposable on their mirror images are chiral.
Reason: All chiral molecules hae chiral centres.AIf both assertion and reason are correct and reason is correct explanation for assertionBIf both assertion and reason are correct but reason is not correct explanation for assertion.CIf assertion is correct but reason is incorrect.Dif both assertion and reason are incorrect.
A carbon atom having four different groups is known as
View SolutionA molecule having a plane of symmetry is a chiral molecule.
View SolutionState True or false
a chiral carbon consists of 4 different groups Attached.
View SolutionAn asymmetric carbon atom is a chiral centre.
View Solution(A) Molecuels that are not superimposable on their mirror images are chiral.
(R) All chiral molecules have chiral centre.
View Solution

