Which one of the following relationships when graphed does not give a straight line for helium gas?
I. K.E. and T at constant pressure and volume
II. P v/s V at constant temperature for a constant mass
III. V v/s 1/T at constant pressure for a constant mass
A
II
B
II and III
C
III
D
I
Video Solution
Text Solution
The correct Answer is:b
(b) KET=32nRT at constant P and V
PV=K at constant T and mass
V=KT at constant P and mass
|
Similar Questions
Knowledge Check
What is the nature of graph of PV versus P for a given mass of a gas at constant temperature?
AParallel to X-axisBParallel to Y-axisCLinear with positive slopeDLinear with negative slopeFor a given mass of a gas at constant temperature, if the volume V becomes three times, then the pressure (p) will become :
A3pBp/3C3p/TD9p2which of the following graphs correctly represents the vatiation β=−(dVdP)T/V with P for an ideal gas at constant temperature ? .
A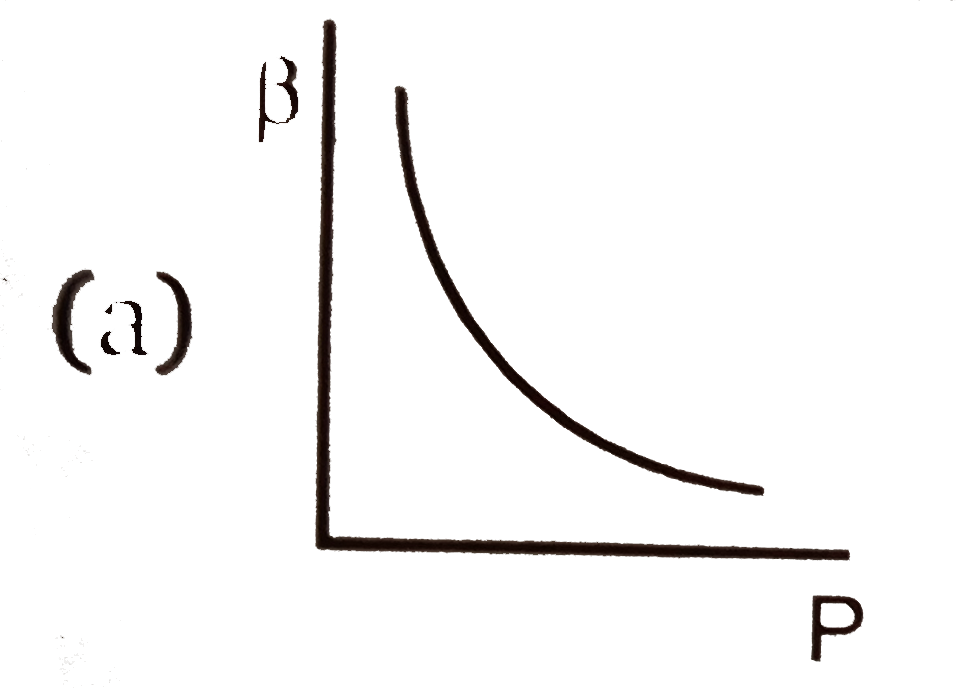 B
B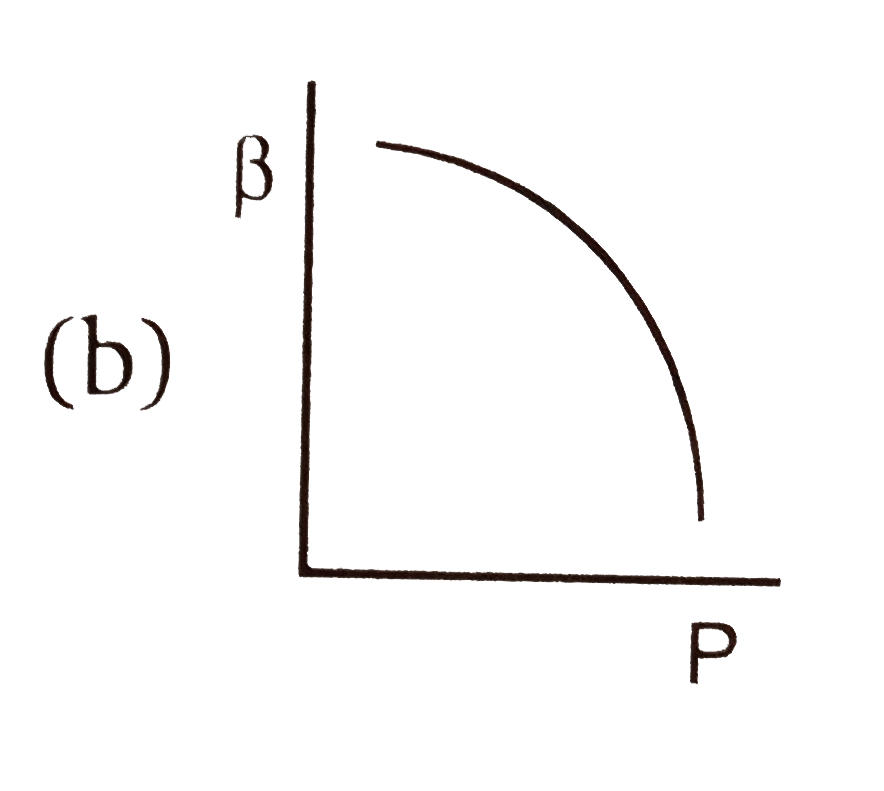 C
C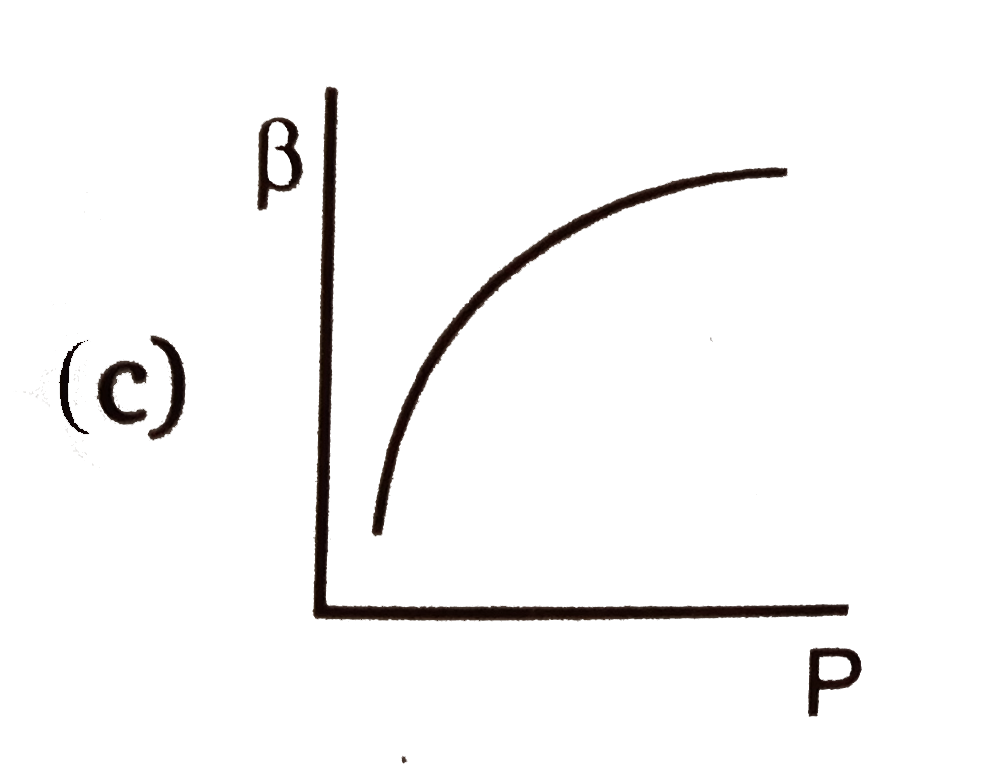 D
D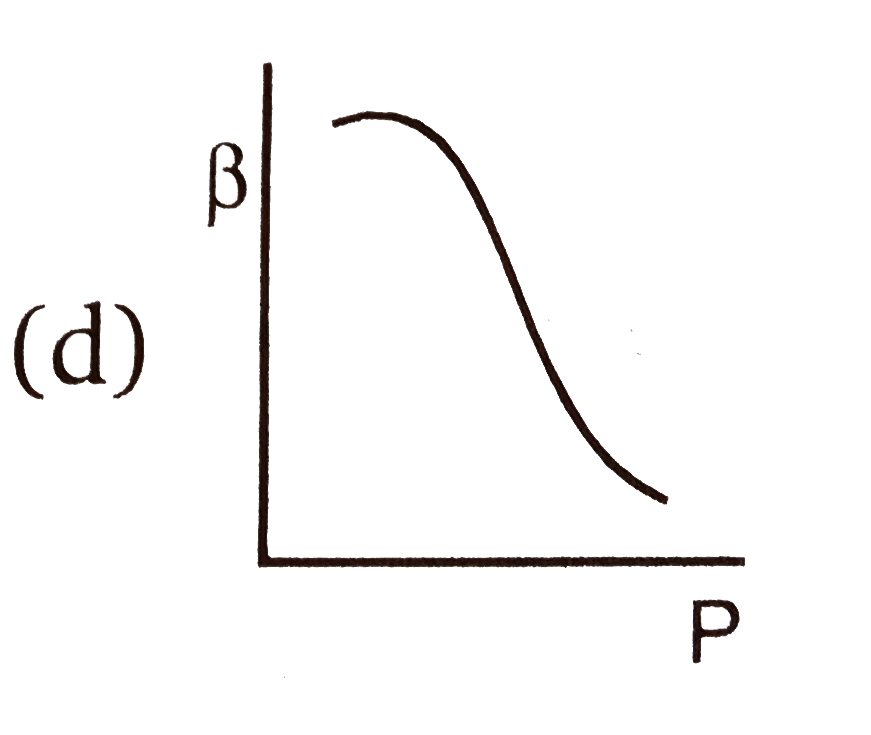
if pressure P is inversely proportional to volume (V) at constant temperature, then PV is constant (True/False)
View SolutionFor a given mass of a gas at constant temperature,if the volume,'V' becomes three times then pressure P will become
View SolutionIf Cp and Cv are molar heats at constant pressure and constant volume respectively and R is gas constant for 1 mole, then the correct relation is
View SolutionIf P, V, and d are the pressure, Volumne and density of a given mass of gas at constant temperature, choose the correct option.
View SolutionUnder constant pressure, graph between p and 1V is a
View Solution

