What are the postulates of Bohr's model of hydrogen atom ? Discuss the importance of this model to explain various series of line spectra in hydrogen atom. Solution in Telugu
Postulates of Bohr's theory :
(1) Electrons revolve around the uncleus in certain , definite circular paths , called orbits . They are also known as energy levels or shells .
(2) Each orbit is associated with a definite amount of energy . Hence these orbits are called energy orbits or energy levels or energy shells .
(3) As the value of n increases , the size and energy of the orbit increases .
(4) As long as the electrons revolve in the circular orbits , they neither emit nor absorb energy . Hence , these orbits are also called stationary orbits .
(5) More than one stationary orbit is possible for any electron and the angular momentum of the revolving electron is quantized . That is , the electrons revolve in those circular orbits in which the angular momentum of the revolving electron is an integral multiple of h/2πr .
This can be expressed as mvr=n×h2π
where m = mass of the electron,
v = velocity of the electron
r = radius of the orbit ,
h = Planck's constant ,
n = Principal quantum number .
(6) The energy of electron changes when the electron jumps from one orbit to another orbit . When the electron jumps from a lower orbit to a higher orbit , energy is absorbed . Similarly when the electron jumps from a higher orbit to a lower orbit , erergy is emitted . The energy emitted or absorbed is given by the equation , Δ=E2−E1=hυ
where
E1 = Energy of lower orbits ,
E2 = Energy of higher orbits ,
ΔE = Difference in energy ,
h = Planck's constant,
υ = Frequency.
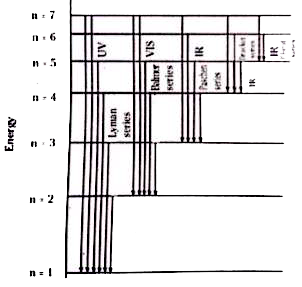
(B) Explanation of formation of different lines in various series of Hydrogen atomic spectrum : Hydrogen atom has only one electron and it revolves around the uncleus in the first orbit . When certain amount of hydrogen gas containing a large number of electrons , is heated or exposed to light energy or is subjected to electric discharge the different electrons absorb different amount of energy and get excited to different higher orbits. But , they do not stay there for longer peridos . Hence , they try to come back to lower orbits (de-excitation ) . This de - excitation need not necessarily be the same for all the atoms . Some excited electrons may come back from any of the hight energy levels to lower energy level n = 1 . Then Lyman series is formed in the U.V. region . Some excited electrons may come back from any of the higher energy levels to lower energy level n = 2 . Then Balmer series is formed in the visible region . Similarly , when the excited electron comes back , from any of the higher energy levels to lower 3 , 4 & 5 , we get Paschen , Bracket and Pfund series respectively in the I.R . region.
The de-excitation process from higher energy level to lower energy level also may take place in a single step or in different steps . For example , the de-excitation process of an electron from 4th energy level to 1st energy level can be as shown below.
Direct step : From 4th level to 1st (i.e.,) 4→1
Different steps : 4→3→2→1,4→3→1,4→2→1.
For each electronic transition one line is formed in the spectrum . Thus we get a large number of lines in a given series .
Similar Questions
Question based on Photoelectric Effect | Atomic spectra of Hydrogen | Bohr's model of Atom| Bohr's postulates and derivation of radius and velocity of Bohr's velocity
View SolutionExplain the postulates of Bohr's atomic model.
View SolutionAtomic spectra of hydrogen | Bohr's model of atom | Bohr's postulates | Derivation of radius and velocity of Bohr's orbit
View SolutionPhotoelectric Effect Questions | Atomic spectra of Hydrogen Questions | Bohr's model of Atom| Bohr's postulates and derivation of radius and velocity of Bohr's velocity
View SolutionWriter Bohr's postulates for the hydrogen atom model.
View SolutionWrite two important limitations of Rutherford model which could not explain the observed feature of atomic spectra . How were these explain in Bohr's model of hydrogen atom ?
View SolutionWrite any postulate of the Bohr's model of the hydrogen atom
View SolutionState Bohr's postulates regarding Bohr's model of the hydrogen atom.
View Solution

