Explain diagrammatically the boundary surfaces for three 2p orbitals and five 3d orbitals. Solution in Telugu
Video Solution
Text Solution
Shape of p - orbital : Shape of p -orbital is dumb-bel . There are three 2p - orbitals . They are 2px,2pyand2pz orbitals . Each orbital has two lobes . These lobes are oriented along their respective axes . Each p orbital has one nodal plane.
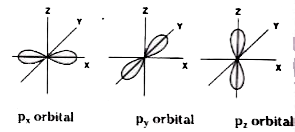
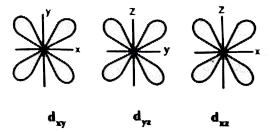
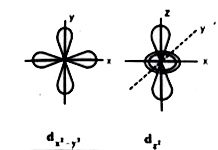
Shape of d-orbital : d -orbital has double dumb -bell shape . There are five d-orbit als. They dxy,dxz,dx2−y2anddz2.
Each d orbital has 4 lobes . In dxy orbital , the lobes are placed in- between the x and y axes, similar is the case with other orbitals, dyzanddxz. In dz2 orbital , two lobes lie along z aixs and there is a ring of electron-cloud around the centre. In dx2−y2 orbital , the lobes lie along x and y axes. Each d orbital has two nodal planes.
|
Similar Questions
" The number of angular nodes in "2p" and "3d" orbitals respectively are "
View SolutionWhat is orbital ? Explain point and boundary surface diagram of orbitals
View SolutionExplain 2p orbitals
View SolutionWhat are the values of the orbital angular momentum of an electron in the orbitals 1s, 3s, 3d and 2p ?
View SolutionSketch the boundary surface diagrams of 2p orbitals.
View SolutionWhat is orbital? Draw the shapes of 2p and 3d orbitals.
View SolutionA : The orbitals having equal energy are known as degenerate orbitals
R : The three 2p orbitals are degenerate in the presence of external magnetic field .
View SolutionExplain diagrammatically the boundary surfaces for three 2p orbitals and five 3d orbitals.
View Solution

