Write a note on
(c ) Covalent radius.Solution in Telugu
Video Solution
Text Solution
Atomic radius refer to both metallic radius and covalent radius.
(c ) Covalent radius: It is defined as one half of the equilibrium distance between the nuclei of two atoms bonded by a covalent bond.
Ex. Covalent radius of hydrogen = 0.37 Å
Covalent radius of chlorine = 0.99 Å
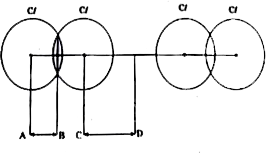
AB = covalent radius = 0.99 Å
CD = van der Waal's radius = 1.8 Å
Covalent,van der Waal's radii of cholrine.
van der Waal's radius: It is defined as one-half of the inter-nuclear distance between atoms of two adjacent molecules of an element bonded by van der Waal's forces facing each other, in solid state.
Ex. van der Waal's radius of hydrogen = 1.2 Å
van der Waal's radius of chlorine = 1.80 Å
Note: van der Waal's radius of an atom is 40% larger than its covalent radius.
|
Similar Questions
Write the short note on radius of gyration.
View SolutionWrite short notes on: Covalent bond
View SolutionWrite note on covalent network crystals.
View SolutionWrite short notes on APC/C.
View SolutionWrite short notes on (i) Covelent radius
View SolutionWrite short notes on (ii) Vander Waal's radius.
View SolutionWrite short notes on (iii) Ionic radius
View SolutionWrite a note on
(a) Atomic radius.
View Solution

