Oxalic acid is oxidised by permanganate ion in acid medium of Mn2+ balance the reaction by ion-electron method.Solution in Telugu
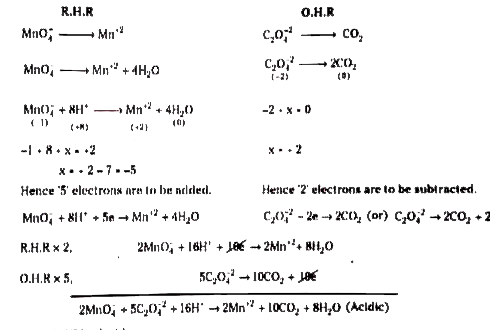
Adding 16OH− both sides
2MnO−4+5C2O−24+16H++16OH−→2Mn+2+10CO2+8H2O+16OH−
2MnO−4+5C2O−24+16H2O→2Mn+2+10CO2+8H2O+16OH−
2MnO−4+5C2O−24+8H2O→2Mn+2+10CO2+16OH− (Basic)
Similar Questions
ఆమ్లయానకంలో ఆక్జాలిక్ ఆమ్లం, పర్మాంగనేట్ అయాన్ తో Mn2^+ గా అక్సీకరించబడుతుంది. అయాన్ -ఎలక్ట్రాన్ పద్ధతిలో తుల్యం చేయండి.
View SolutionWrite balanced chemical equations for the following reactions :
(i) Permanganate ion (MnO−4) reacts with sulphur dioxide gas in acidic medium to produce Mn2+ and hydrogen sulphate ion (Balance by ion electron method)
(ii) Reaction of liquid hydrazine (N2H4) with chlorate ion (ClO−3) in basic medium produces nitric oxide gas and chloride ion in gaseous state. (Balance by oxidation number method)
(iii) Dichlorine heptaoxide (Cl2O7) in gaseous state combines with an aqueous solution of hydrogen peroxide in acidic medium to give chlorite ion (ClO−2) and oxygen gas , (Balance by ion electron method)
View SolutionWrite balanced chemical equation for the following reaction.
(a) Permanganate ion (MnO−4) reacts with sulphur dioxide gas in acidic medium to produce Mn+2 and hydrogen sulphate ion. (Balance by ion electron method)
(b) Reaction of liquid hydrazine (N2H4) with chlorate ion (ClO−3) in basic medium Produces nitric oxide gas and chloride ion in gaseous state. (Balance by oxidation in the following species. number method)
( c) Dichlorine heptaoxide (Cl2O7) in gaseous state combines with an aqueous solution of hydrogen peroxide in acidic medium to give chlorite ion (ClO−2) and oxygen gas. (Balance by ion electron method)
View SolutionWrite the balanced equation of the following reactions. Permanganate ions in acidic medium (MnO_4^(-)) , With sulfur dioxide gas Mn^(2+) And gives hydrogen sulfate ion, (balance ion by electron method)
View SolutionBalance the following redox reactions by ion electron method MnO_4^-(aq) + SO_2 (g) to Mn^(2+) (aq) + HSO_4^- (aq) (acid medium)
View Solutionoxidation & reduction,oxidising agent and reducing agent,redox reaction and their types,balancing OF redox reaction by ion-electron method in acidic medium
View SolutionBalance the following equations by Ion-Electron method. Mno_4^- +Br^- + H^+ rarr Mn^(+2) +Br_2+H_2O (in acidic medium)
View SolutionBalancing the following redox reactions by ion-electron method. \(MnO_{4}^{-1}+SO_{2} (g) \rightarrow Mn^{2+}+HSO_{4}^{-}\) ( in Acid medium )
View Solution
