Balance the following equation by the oxidation number method.
MnO2−4+Cl2→MnO2−4+Cl−Solution in Telugu
Step - I : The skeleton reaction
MnO2−4+Cl2→MnO−4+Cl−
Step - II : 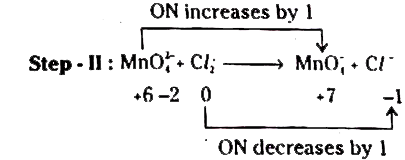
Step - III : Equalise the increase/decrease in ON. Here they are equal.
Step - IV : Balance the other atoms except H and O
2MnO2−4+Cl2→2MnO−4+2Cl−
Step - V : Balance H atoms O atoms. Here they are balanced.
The balanced equation is
2MnO2−4+Cl2→2MnO−4+2Cl−
Similar Questions
Balance the following equation by oxidation number method. KMNO_(4)+HClrarrKCl+MnCl_(2)+H_(2)O+Cl_(2)
View SolutionBalance the following equations by the oxidation number method. a) Cr_((s))+Pb(NO_(3))_(2(aq))rarr Cr(NO_(3))_(3(aq))+Pb_((s)) b) MnO_(4)^(-2) +Cl_(2)rarr MnO_(4)^(-)+Cl^(-)
View Solutionनिम्न अभिक्रिया को ऑक्सीकरण अंक विधि द्वारा संतुलित कीजिए।
MnO−4+Fe−2→Mn+2+Fe+3
View SolutionBalance the following equations by oxidation number method. MnO_(4)^(-)+H_(2)O_(2)+H^(+)toMn^(2+)+H_(2)O+O_(2)
View SolutionBalance the following equations by oxidation number method. MnO_(2)+HCl toMnCl_(2)+Cl_(2)+H_(2)O
View SolutionMnO_(4)^(-) is a good oxidising agent in different mediums changing to MnO_(4)^(-)toMn^(2+) toMnO_(4)^(2-) toMnO_(2) toMn_(2)O_(3) Changes in oxidation number respectively are
View SolutionBalance the following redox reactions by ion-electron method.
MnO−4(aq)+I−(aq)→MnO2(s)+I2(s) (in basic medium)
View SolutionBalance the following reaction by the oxidation number method - MnO_(4)^(-)+Fe^(+2)rarrMn^(+2)+Fe^(+3)
View Solution

