Explain aromatic electroplhilic substitution reactions of benzene.Solution in Telugu
Video Solution
Text Solution
Electrophilic substitution reaction of benzene proceeds in two steps.
Step 1: The clectrophile (E') takes two electrons of the six - electron π system, to form a σ bond with a carbon atom of the benzene ring. Then that carbon atom becomes sp3 hybridised.

Step 2: A proton is removed from the carbon atom of the σ complex that bears the E (electrophile). Then that carbon atom changes to sp2 state again. Then the benzene derivative with fully delocalised six π electrons is formed.
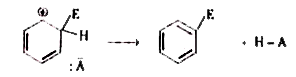
(The proton H∗ combines with the anion obtained from the molecule E−A→E∗+:A)
|
Similar Questions
Explain the aromatic electrophilic substitution reactions of anisole with equations. Aromatic electrophilic substitution reactions:
View SolutionFor an electrophilic aromatic substitution reaction :
View SolutionDescribe the aromatic electrophilic substitution action of benzene.
View SolutionWhat is substitution reaction? Explain any two substitution reactions of benzene.
View Solution(A) Fridel-Crafts acylation gives an aromatic ketone from benzene
(R) : Acylation reaction is a uncleophilic substitution reaction with respect to benzene .
View Solutionइलेक्ट्रोफिलिक एरोमेटिक प्रतिस्थापन अभिक्रिया के लिए
View SolutionReaction OF Aromatic Benzene Ring
View Solution

