The hybridisation of Xe and the number of lone pairs of electrons on it in XeF6 areSolution in Telugu
A
sp3d2,1
B
sp3d3,2
C
sp3d2,2
D
sp3d3,1
Video Solution
Text Solution
The correct Answer is:D
XeF6 has sp3d3 hybridisation, octahedral structure in which six positions are occupied by fluorine atoms and one lone pair is present on the central atom i.e., Xe.
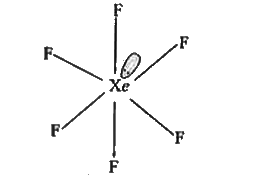
|
Similar Questions
The number of lone pairs of electrons present on Xe in XeF4 is
View SolutionThe number of lone pair of electrons on the Xe - atom in XeF2,XeF4 and XeF6 molecules are respectively -
View SolutionThe hybridisation of Xe and the number of lone pairs of electrons on it in XeF_(6) are
View SolutionIn XeF_(2), XeF_(4) and XeF_(6) the number of lone pairs on Xe is respectively.
View SolutionNumber of lone pairs electrons on Xe atoms in XeF_(2), XeF_(4) and XeF_(6) molecules are respectively.
View SolutionIn XeF_(2),XeF_(4)" and "XeF_(6), the number of the lone pairs of Xe respectively are
View SolutionIn XeF_(2) , xeF_(4) and XeF_(6) the number of lone pairs and Xe is respectively.
View Solution
