(a) Electropositive nature of the element(s) increases down the group and decreases across the period
(b) Electronegativity of the element decreases down the group and increases across the period
(c) Atomic size increase down the group and decreases across a period (left to right)
(d) Metallic character increases down the group and decreases across a period
On the basis of the above trends of the periodic table, answer the following about the electron with atomic number 3 to 9
(a) Name the most electropositive element among them
(b) Name the most electronegative element
(c) Name of the element with smallest atomic size
(d) Name the element which is a metalloid
(e) Name the element which shows maximum valency.
The names and symbols of elements having atomic numbers 3 - 9 are
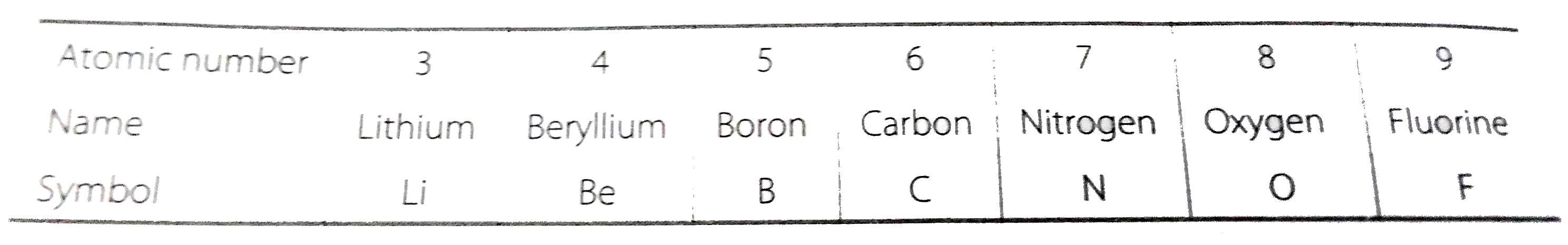
(a) Most electropositive element is lithium (Li)
(b) Most electronegative elemento is fluorine (F)
(c) Element with smallest atomic size is fluorine (F)
(d) Metalloid element is boron (B)
(e) Carbon (C) shows maximum valency i.e., 4.
Similar Questions
(a) Electropositive nature of the element(s) increases down the group and decreases across the period.
(b) Electronegativity of the element decreases down the group and increases across the period.
(c) Atomic size increases down the group and decreases across a period (left to right).
(d) Metallic character increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
Name the most electropositive element among them.
View Solution(a) Electropositive nature of the element(s) increases down the group and decreases across the period
(b) Electronegativity of the element decreases down the group and increases across the period
(c) Atomic size increase down the group and decreases across a period (left to right)
(d) Metallic character increases down the group and decreases across a period
On the basis of the above trends of the periodic table, answer the following about the electron with atomic number 3 to 9
(a) Name the most electropositive element among them
(b) Name the most electronegative element
(c) Name of the element with smallest atomic size
(d) Name the element which is a metalloid
(e) Name the element which shows maximum valency.
View Solution
Knowledge Check
Assertion : Acidity of hydrides of group 16 elements increases down the group.
Reason : Electronegativity of these elements increases down the group.AIf both assertion and reason are correct and reason is correct explanation for assertion.BIf both assertion and reason are correct but reason is not correct explanation for assertion.CIf assertion is correct but reason is incorrect.DIf both assertion and reason are incorrect.
(a) Electropositive nature of the element(s) increases down the group and decreases across the period.
(b) Electronegativity of the element decreases down the group and increases across the period.
(c) Atomic size increases down the group and decreases across a period (left to right).
(d) Metallic character increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
Name the most electronegative element.
View Solution(a) Electropositive nature of the element(s) increases down the group and decreases across the period.
(b) Electronegativity of the element decreases down the group and increases across the period.
(c) Atomic size increases down the group and decreases across a period (left to right).
(d) Metallic character increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
Name the element with smallest atomic size.
View Solution(a) Electropositive nature of the element(s) increases down the group and decreases across the period.
(b) Electronegativity of the element decreases down the group and increases across the period.
(c) Atomic size increases down the group and decreases across a period (left to right).
(d) Metallic character increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
Name the element which is a metalloid.
View Solution(a) Electropositive nature of the element(s) increases down the group and decreases across the period.
(b) Electronegativity of the element decreases down the group and increases across the period.
(c) Atomic size increases down the group and decreases across a period (left to right).
(d) Metallic character increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
Name the element which shows maximum valency.
View Solution(i) Electropositive nature of the element(s) increases down the group and decrease across the period.
(ii) Electronegativity of the element decreases down the group and increases across the period.
(iii) Atomic size increases down the group and decrease across the period.
(iv) Metallic character increases down the group and decreases across the period.
On the basis of the above trends of the periodic table, answer the following about the elements with atomic number 3 to 9.
Name the most electronegative among them.
View Solution(i) Electropositive nature of the element(s) increases down the group and decrease across the period.
(ii) Electronegativity of the element decreases down the group and increases across the period.
(iii) Atomic size increases down the group and decrease across the period.
(iv) Metallic character increases down the group and decreases across the period.
On the basis of the above trends of the periodic table, answer the following about the elements with atomic number 3 to 9.
Name the element with smallest atomic size.
View Solution(i) Electropositive nature of the element(s) increases down the group and decrease across the period.
(ii) Electronegativity of the element decreases down the group and increases across the period.
(iii) Atomic size increases down the group and decrease across the period.
(iv) Metallic character increases down the group and decreases across the period.
On the basis of the above trends of the periodic table, answer the following about the elements with atomic number 3 to 9.
Name the element which is a metalloid.
View Solution
