For an electrophilic substitution reaction , the presence of a halogen atom in the benzene ring …..
deactivates the ring by inductive effect
deactivates the ring by reasonance
increases the charge density at ortho and para position relative to meta position by resonance.
directs the incoming electrophile to meta position by increasing the charge density relative to ortho and para position.
The correct Answer is:A, C
For an electrophilic substitution reaction, the presence of halogen atom in the benzen ring deactivates the ring by inductive effect and increases the charge density at ortho and para position relative to meta position by resonance.
When chlorine is attached to benzene ring chlorine being more electronegative pulls the electronei.e. , -I-effect.The electron cloud of benzene is less dense. Chlorine makes aryl halide, moderately deactivating group. But due to resonance the electron density on ortho and para position is greater than in meta position
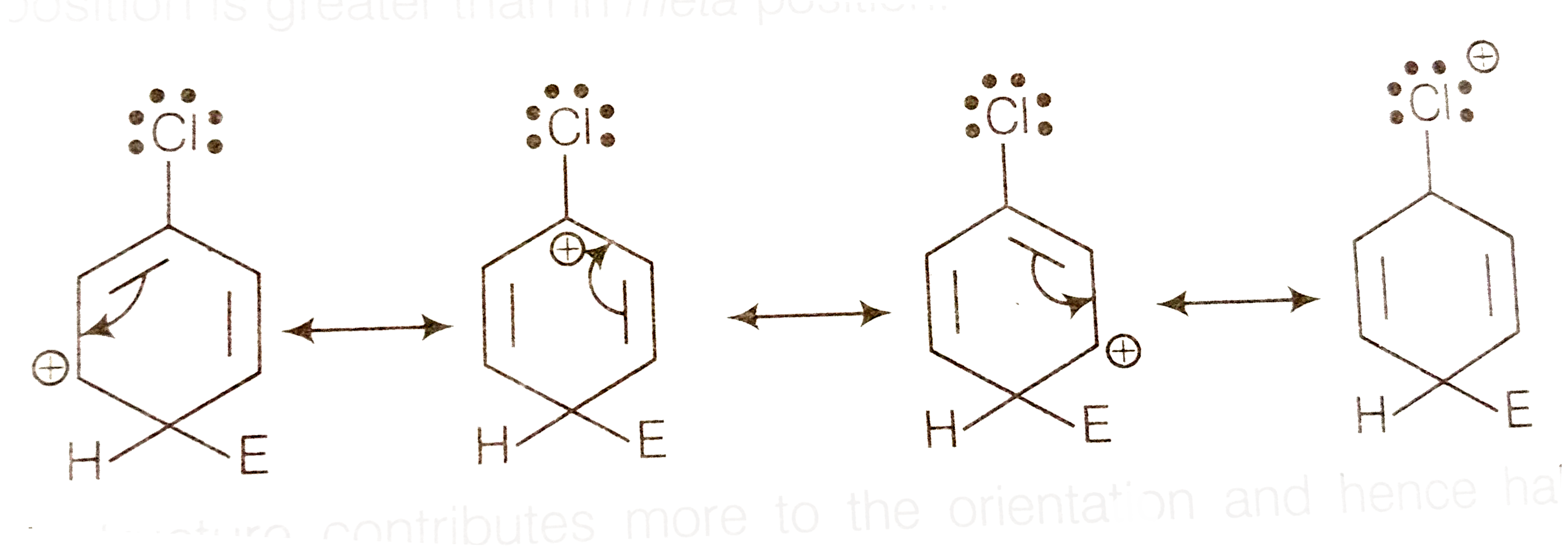
The last structure contributes more to the orientation and hence halogen are o-and p-directors.
Similar Questions
Which of the following reactions is not an example of electrophilic substitution in benzene ring?
View SolutionWhich of the following reactions is not an example of electrophilic substitution in benzene ring?
View SolutionWhat is function of Halogen carrier in direct halogenation of Benzene ring (or in electrophilic substitution of arenes) Explain with mechanism.
View SolutionIn electrophilic substitution reactions of haloarenes, halogen atom is
View SolutionThe typical reaction of benzene and other aromatic compounds is electrophilic substitution. Presence of electron donating group activates the ring towards electrophilic substitution, while presence of electron withdrawing group deactivates the ring towards electrophilic substitution but at the same time activates the ring towards nucleophilic substitution. Some groups are predominantly meta directing and all of these are deactivating. Except halogen, most of the o- and p- directing groups are activating groups. (E.A.S = Electrophilic aromatic substation) The reaction of toluene with Cl_2 in presence of FeCl_3 gives predominantly:
View SolutionThe typical reaction of benzene and other aromatic compounds is electrophilic substitution. Presence of electron donating group activates the ring towards electrophilic substitution, while presence of electron withdrawing group deactivates the ring towards electrophilic substitution but at the same time activates the ring towards nucleophilic substitution. Some groups are predominantly meta directing and all of these are deactivating. Except halogen, most of the o- and p- directing groups are activating groups. (E.A.S = Electrophilic aromatic substation)
Presence of nitro group in benzene ring:
View Solutionइलेक्ट्रॉनरागी विस्थापन अभिक्रियाओं में बेन्जीन वलय में हैलोजन परमाणु की उपस्थिति __________|
View SolutionFor an electrophilic substitution reaction, the presence of a halogens atom in the benzene ring.
View Solution
