Arrange the following set of compounds in the order of their decreasing relative reactivity with an electrophile . Give reason.
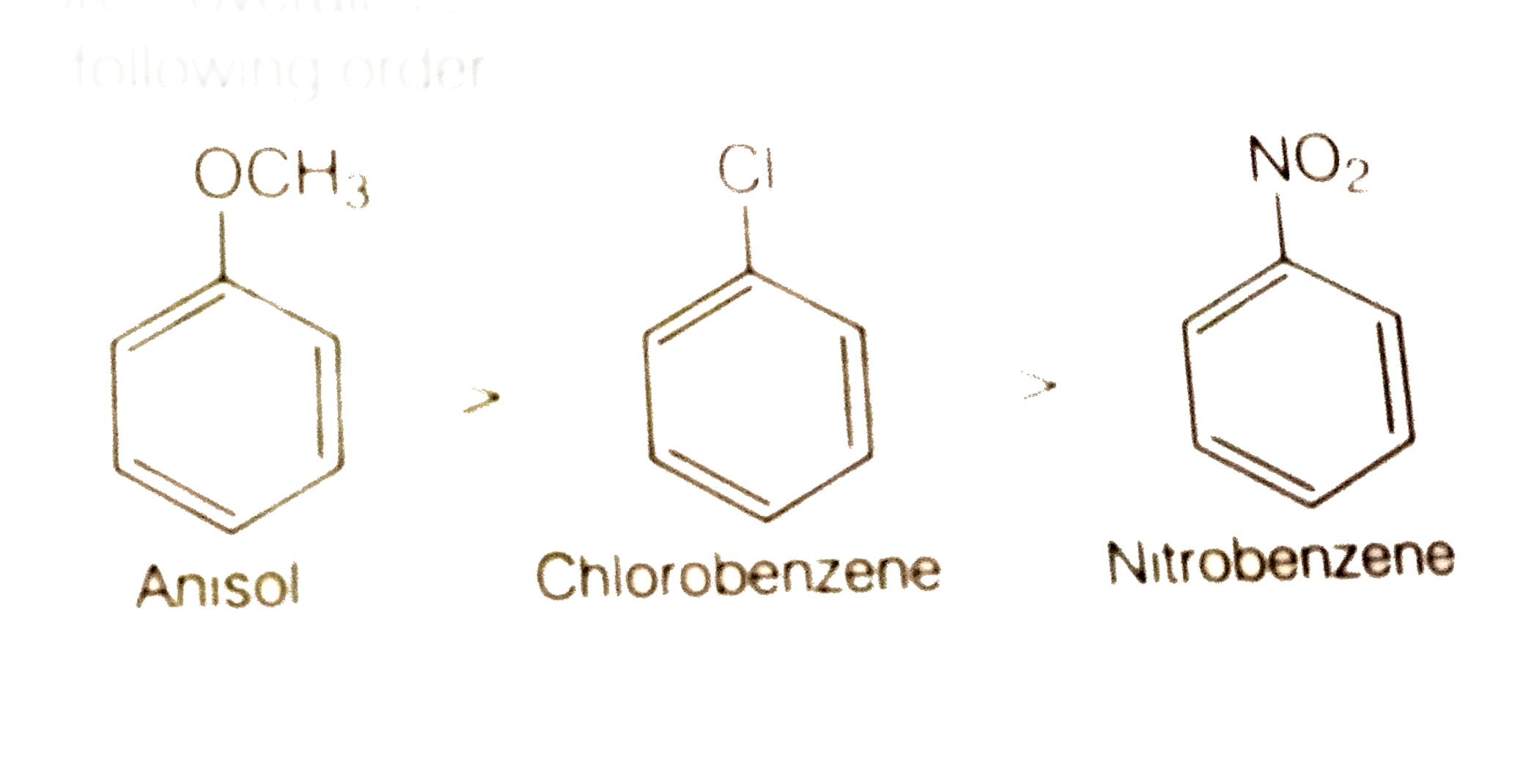
The methoxy group(−OCH3) is electon releasing group. It increases the electron density in benzene nucleus due to resonance effect (+R-effect). It makes anisole more reactive than benzene towards electrophile.

In case of alkyl halides , halogens are moderately deactivating because of their strong -I effect. Thus, overall electron density on benzene ring decreases. It makes further substitution difficult.
−NO2 group is electron withdrawing group. It decreases the electron density in benzene nucleus due to its strong - R- effect and strong -I-effect. Hence, it makes nitrobenzene less reactive. Therefore , overall rearll reactivity of these three compounds towards electrophiles decreases in the following order

Similar Questions
Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+
(a) Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene
(b) Toluene, p−H3C–C6H4–NO2,p−O2N–C6H4–NO2.
View SolutionArrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+
(a) Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene
(b) Toluene, p−H3C–C6H4–NO2,p−O2N–C6H4–NO2.
View Solution
Knowledge Check
Arrange the following set of compounds in the order of their decreasing relative reactivity with an electrophileE+
I.Chlorobenzene
II.2,4 dinitrochlorobenzene
III.P-nitrochlorobenzene
IV.toluene V.p−H3C−C6H4−NO2AI>II>III>IV>VBIV>V>I>III>IICV>IV>III>II>IDI>III>II>V>IVArrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+
(a) Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene
(b) Toluene, p−H3C–C6H4–NO2,p−O2N–C6H4–NO2.A(iii)>(ii)>(i)B(ii)>(iii)>(i)C(i)>(iii)>(ii)D(i)>(ii)>(iii)Arrange the following compounds in decreasing order of their acidic strength.
 AIV<II<I<IIIBI<II<III<IVCIV<II<III<IDI<III<II<IV
AIV<II<I<IIIBI<II<III<IVCIV<II<III<IDI<III<II<IV
Arrange the following compounds in order of their decreasing reactivity with an electrophile,E(+)
i) Chlorobenzene
ii) 2,4- dinitrochlorobenzene
iii)p- nitrochlorobenzene
View SolutionArrange the following compounds in decreasing order of their basicity?
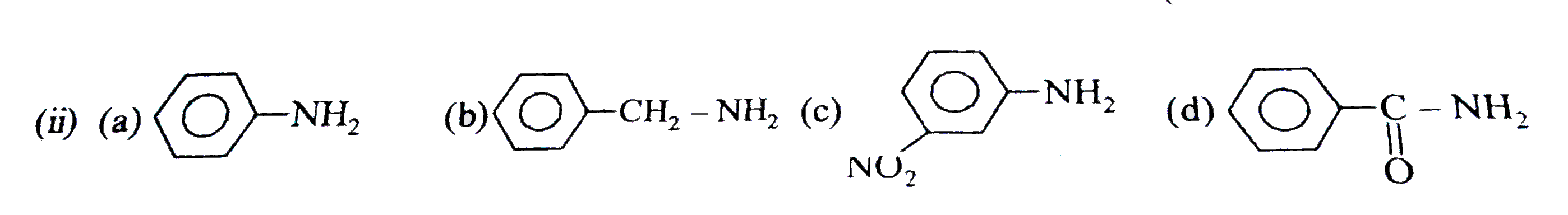 .
.
View SolutionArrange the following compounds in order of their decreasing reactivity with an electrophile,E(+)
i) Chlorobenzene
ii) 2,4- dinitrochlorobenzene
iii)p- nitrochlorobenzene
View SolutionArrange the following compounds in order of decreasing reactivity towards SN1 reaction
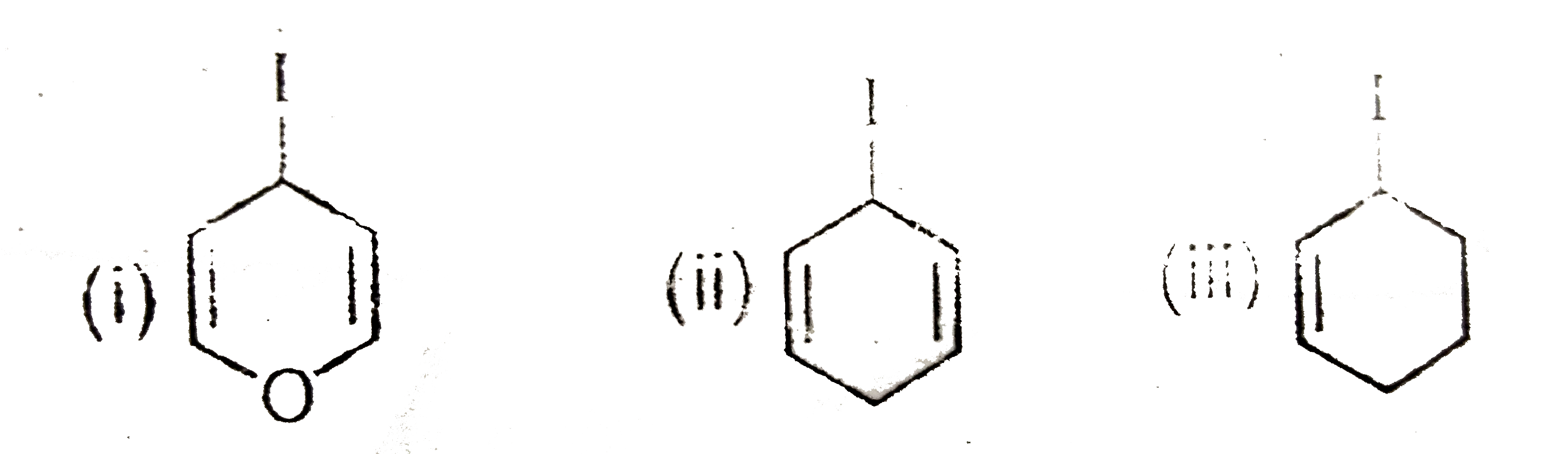
View SolutionArrange the following compounds in order of decreasing acidic strength.
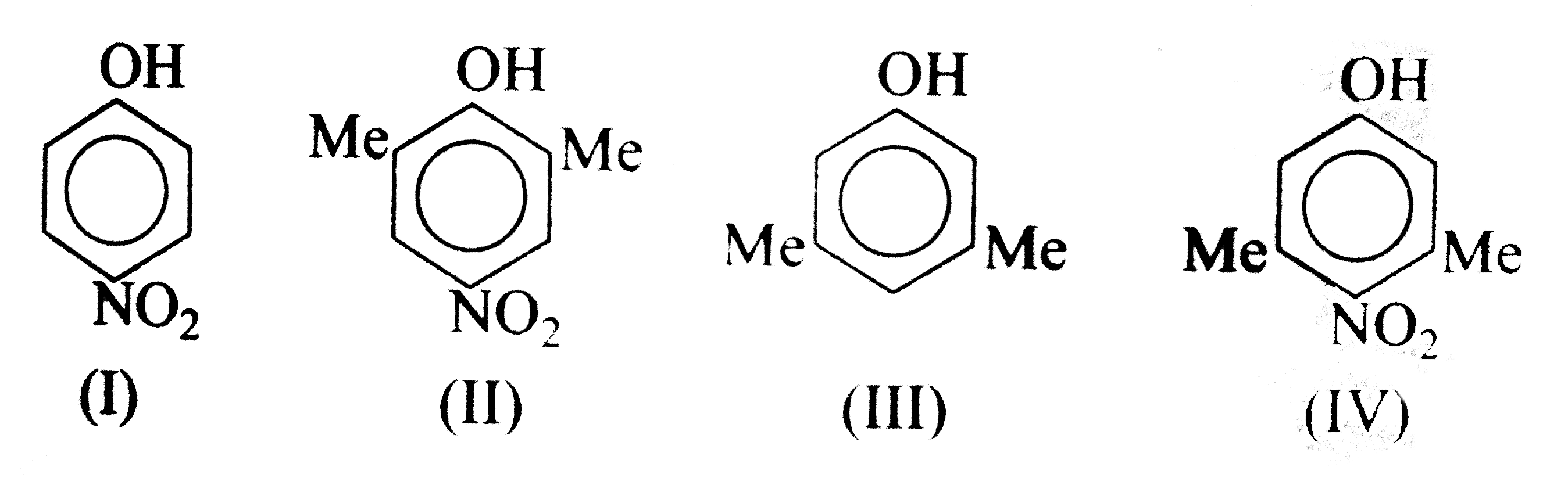
View Solution
