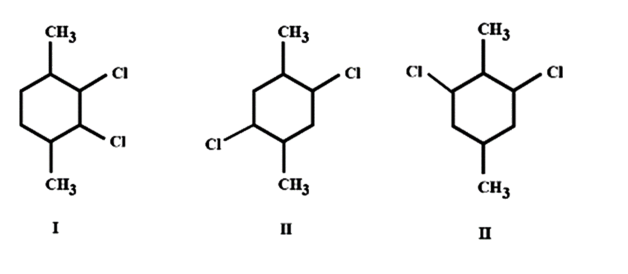
Which of the following compounds will have the highest melting point and why?
Video Solution
Text Solution
In compound (II), both the CH3 groups and Cl atoms at para-position to each other. Therefore, compound (II) is more symmetrical and it fits in the crystal lattice better than the other two isomers and hence it has the highest melting point than the others
|
Similar Questions
Knowledge Check
Which of the following compounds will have highest melting point?
AChlorobenzeneBo-DichlorobenzeneCm-DichlorobenzeneDp-DichlorobenzeneWhich of the following compounds will have the highest melting point here?
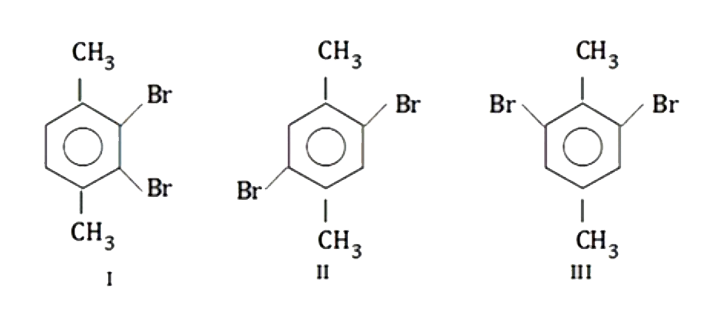 AIIIBIICIDthey have equal melting point
AIIIBIICIDthey have equal melting pointWhich of the following compounds would have the highest boiling point ?
ACH3CH2CH2CH3BCH3NH2CCH3OHDCH2F2
Which of the following compounds would have the highest boiling point ?
View SolutionWhich of the following compounds has the highest melting point
View SolutionWhich will have highest melting point ?
View SolutionWhich will have highest melting point?
View SolutionWhich has the highest melting point?
View Solution