Why is nitric oxide paramagnetic in gaseous state but the solid obtained on cooling it is diamagnetic ?
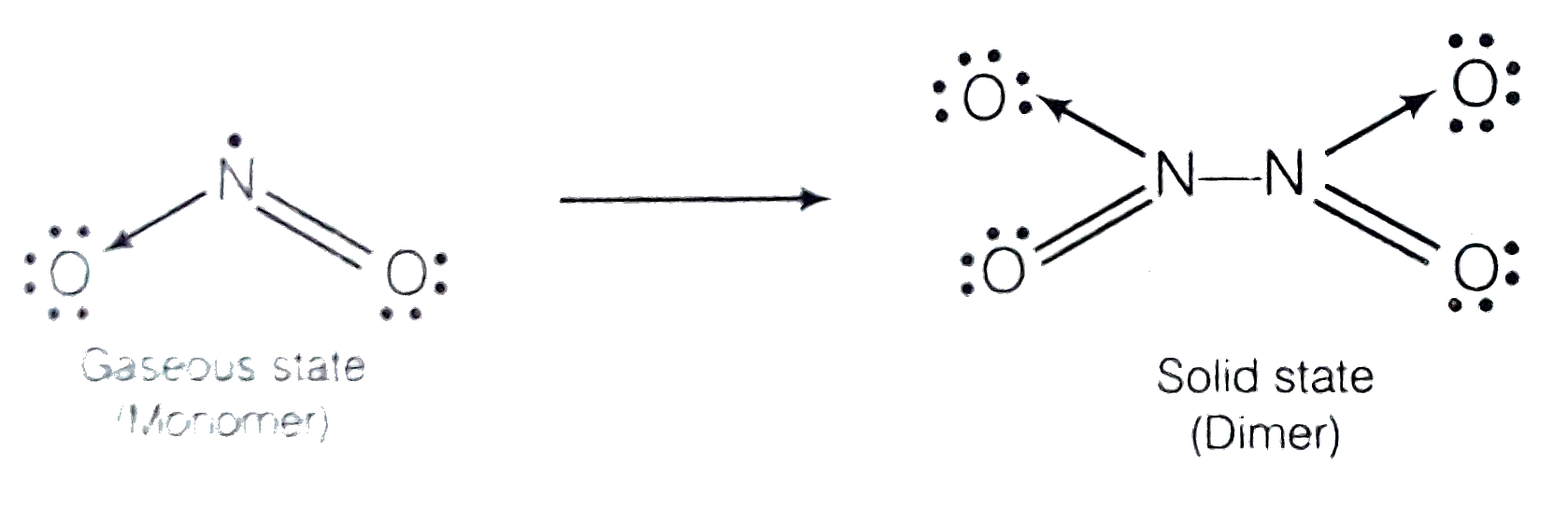
In gaseous state , NO2 exists as a monomer which has one unpaired electron but in solid state, It dimerises to N2O4 so no unpaired electron left. Therefore , NO2 is paramagnetic in gaseous state but diamagnetic in solid state.
Similar Questions
Why is it that nitric oxide is paramagnetic in gaseous state but the solid obtained on cooling it is diamagnetic ?
View SolutionGive reasons for the following :
(i) NO (Nitric oxide) is paramagnetic in the gaseous state but diamagnetic in the liquid and solid states. Why?
(ii) Nitric oxide becomes brown when released in air.
View Solution
Knowledge Check
Nitric oxide (NO) is paramagnetic in gaseous state
Agaseous stateBsolid stateCliquid stateDpolymeric stateThe compound is covalent in gaseous state but ionic in solid state is.
APCl5BPCl3CCCl4DNH3Assertion: Nitric oxide is paramagnetic in the liquid and solid states.
Reason: Nitric oxide is an odd electron molecule and the gas is paramagnetic.ABoth A and R are correct and R is the correct explanation of ABBoth A and R are correct and R is not the correct explanation of ACA is correct R is wrongDA is wrong R is correct
Give reasons for the following :
(i) NO (Nitric oxide) is paramagnetic in the gaseous state but diamagnetic in the liquid and solid states. Why?
(ii) Nitric oxide becomes brown when released in air.
View SolutionWhy is HCl predominantly covalent in the gaseous state but is ionic in squeous solution ?
View SolutionThe conversion of a liquid to its solid state on cooling is called __________ .
View SolutionWhy are ionic solids conducting in the molten state and not in the solid state ?
View SolutionWhy is HCl predominantly covalent in the gaseous state but ionic in the aqueous solution?
View Solution
