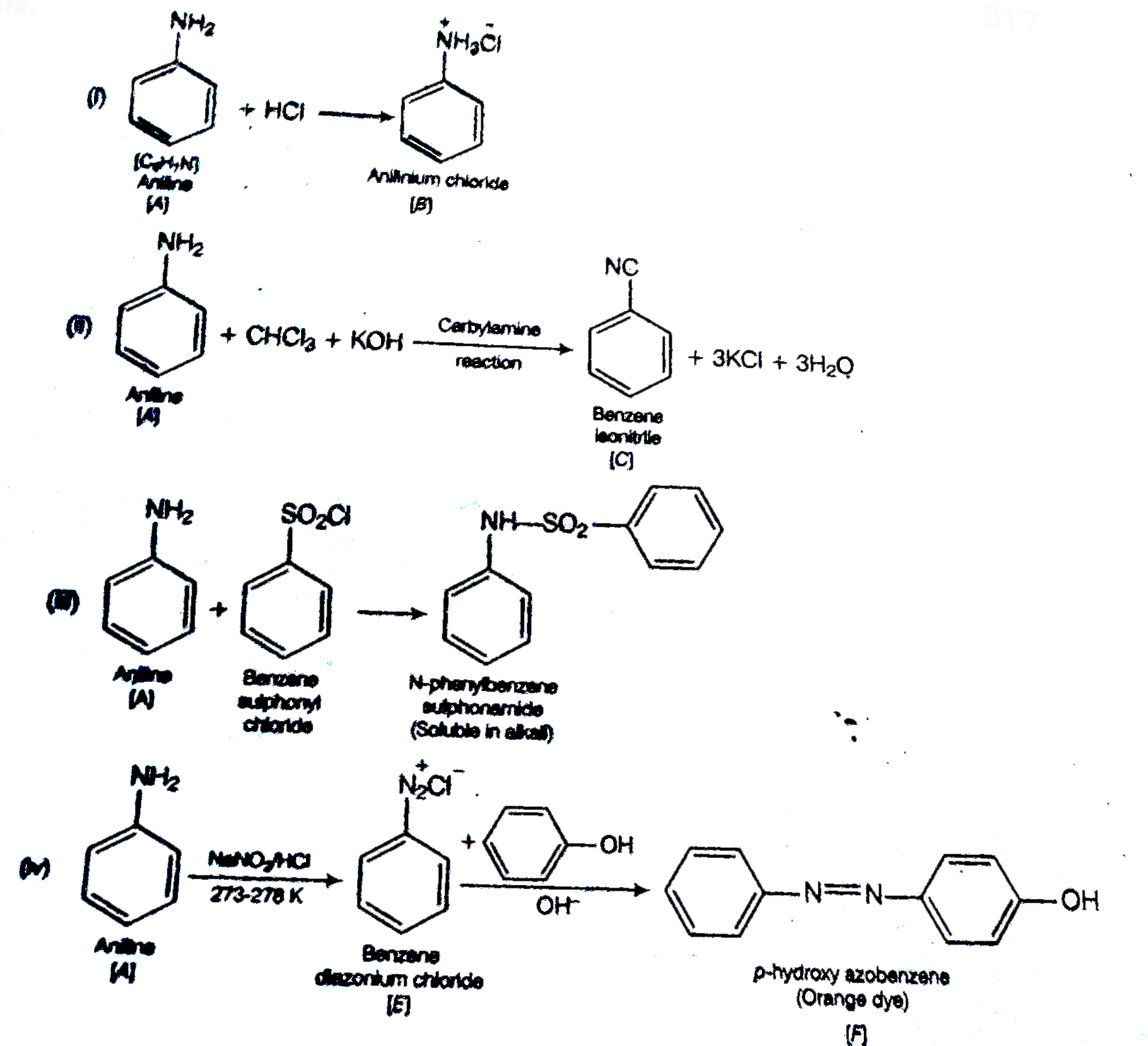A colours substance 'A' (C6H7) is sparingly soluble in water and gives a water soluble comound 'B' on treating with mineral acid. On reaction wih CHCl3 and alcoholic potash 'A' produces an obnoxious smell due to the formation of compound 'C'. Reaction of 'A' with benzensulphonyl chloride gives compound 'D' which is soluble in alkali. WIth NaNO2 and HCl, 'A' forms compound 'E' which reacts with phenol in alkaline medium to give an orange dye 'F'. Identify compounds 'A' to'F'.
Similar Questions
A colours substance 'A' (C6H7N) is sparingly soluble in water and gives a water soluble compound 'B' on treating with mineral acid. On reaction wih CHCl3 and alcoholic potash 'A' produces an obnoxious smell due to the formation of compound 'C'. Reaction of 'A' with benzensulphonyl chloride gives compound 'D' which is soluble in alkali. WIth NaNO2 and HCl, 'A' forms compound 'E' which reacts with phenol in alkaline medium to give an orange dye 'F'. Identify compounds 'A' to'F'.
View SolutionAn organic compound B reacts with Benzene sulphonic chloride to give a compound which is soluble in alkali metal hydroxide. What is B?
View Solution
Knowledge Check
Which compound is soluble in water
ACS2BC2H5OHCCCl4DCHCl3Which compound is soluble in water
ACS2BC2H5OHCCCl4DCHCl3Acetanilde (C6H5NHCOCH3) is sparingly soluble in cold water but readily soluble in boiling water. It is usually purifies by
AchromatographyBcrystallizationCsublimationDdistillation
An organic aromatic compound 'A' with the molecular formula C6H7N is sparingly soluble in water. 'A' on treatment with dil. HCl gives a water soluble compound 'B'. 'A' also reacts with chloroform in presence of alcoholic KOH to form an obnoxious smelling compound 'C'. 'A' reacts with benzene sulphonyl chloride to form an alkali soluble compound 'D'. 'A' reacts with NaNO2 and HCl to form a compound 'E' which on reaction with phenol forms an orange red dye 'F'. Elucidate the structures of the organic compounds from 'A' to 'F'
View SolutionA colourless substance (A) is sparingly soluble in water and gives (B) on heating with mineral acids. Compound (B) on reaction with CHCl3 and alcoholic potash produces an obnoxious smell of carbylamine due to the formation of (C). Compound (A) on reaction with chlorosulphonic acid gives (D) which on treatment with ammonia gives (E). Compound (E) on hydrolysis gives sulphanilamide, a well known drug. Give structures of (A) to (E) with proper reasoning.
View SolutionAn compound react with Hinsberg's reagent gives a product which is soluble in alkali solution (NaOHsolution) compound is :
View SolutionCompound A on reduction gives B which on further reaction with CHCl3 and alcoholic KOH gives compound C which of further hydrolysis gives aniline. The compound A is :
View SolutionWhich of the following compounds is sparingly soluble in ammonia
View Solution