Which of the following would you predict to be the stronger acid ? CH3−CH=CH−CH2−OH or CH3−CH=CH−OH
The correct Answer is:2
To determine which of the two compounds is the stronger acid, we need to analyze the stability of their conjugate bases after the loss of a proton (H⁺).
1. Identify the Compounds:
- Compound 1: CH₃−CH=CH−CH₂−OH
- Compound 2: CH₃−CH=CH−OH
2. Determine the Conjugate Bases:
- For Compound 1, when the hydroxyl group (OH) loses a proton (H⁺), the conjugate base formed is:
- CH₃−CH=CH−CH₂−O⁻
- For Compound 2, when the hydroxyl group (OH) loses a proton (H⁺), the conjugate base formed is:
- CH₃−CH=CH−O⁻
3. Analyze the Stability of the Conjugate Bases:
- Compound 1 Conjugate Base:
- CH₃−CH=CH−CH₂−O⁻ does not have resonance stabilization because the negative charge on the oxygen cannot be delocalized effectively.
- Compound 2 Conjugate Base:
- CH₃−CH=CH−O⁻ can have resonance stabilization. The negative charge on the oxygen can be delocalized into the double bond, allowing for resonance structures that stabilize the conjugate base.
4. Compare the Stability:
- The conjugate base of Compound 2 is more stable due to resonance, while the conjugate base of Compound 1 lacks such stabilization.
5. Conclusion:
- Since the conjugate base of Compound 2 is more stable, we can conclude that Compound 2 (CH₃−CH=CH−OH) is the stronger acid compared to Compound 1 (CH₃−CH=CH−CH₂−OH).
Final Answer:
The stronger acid is CH₃−CH=CH−OH.
- Compound 1: CH₃−CH=CH−CH₂−OH
- Compound 2: CH₃−CH=CH−OH
- For Compound 1, when the hydroxyl group (OH) loses a proton (H⁺), the conjugate base formed is:
- CH₃−CH=CH−CH₂−O⁻
- For Compound 2, when the hydroxyl group (OH) loses a proton (H⁺), the conjugate base formed is:
- CH₃−CH=CH−O⁻
- Compound 1 Conjugate Base:
- CH₃−CH=CH−CH₂−O⁻ does not have resonance stabilization because the negative charge on the oxygen cannot be delocalized effectively.
- Compound 2 Conjugate Base:
- CH₃−CH=CH−O⁻ can have resonance stabilization. The negative charge on the oxygen can be delocalized into the double bond, allowing for resonance structures that stabilize the conjugate base.
- The conjugate base of Compound 2 is more stable due to resonance, while the conjugate base of Compound 1 lacks such stabilization.
- Since the conjugate base of Compound 2 is more stable, we can conclude that Compound 2 (CH₃−CH=CH−OH) is the stronger acid compared to Compound 1 (CH₃−CH=CH−CH₂−OH).
The stronger acid is CH₃−CH=CH−OH.
Similar Questions
Knowledge Check
ClCH2CH2OH is stronger acid than CH3CH2OH because of :
A−I effect of Cl increases negative charge on O atom of alcohol.B−I effect of Cl disperses negative charge on O atom to produce more stable cation.C−I effect of Cl disperses negative charge on O atom to produce more stable anion.DNone of these.
The iupac name of CH3−CH(CH3)−CH2−OH
View SolutionThe IUPAC name of CH3−CH(OH)−CH2−C(OH)(CH3)2 is :
View SolutionCH3−CH2−CH2−CH2−OH→CH3−CH2−CH=CH2+H2O
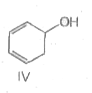
View SolutionWhich of the following is the correct ease of dehydration ?
I CH3−CH2−CH2−CH2∣OH , II CH3−CH2−OH∣CH−CH3 , III CH2=CH−OH∣CH−CH3, IV
View SolutionWhich of the following compounds gives fastest reaction with HBr and why?
(i) (CH3)3_OH (ii) CH3CH2CH2OH
(iii) CH3OH∣CHCH3 (iv) CH3CH3∣CHCH2OH
View SolutionCH3CH(OH)CH2CH2COOH
View SolutionCH3−CH2−CH2−CH2−OH and CH3−C(CH3)2−OH are
View Solution
