Arrange the following compounds in order of increaing basicity.
(a). CH3NH2,CH3NH⊕3,CH3NH−
(b) CH3O−,CH3NH−,CH3CH−2.
The correct Answer is:1<2<3
Step by step video & text solution for Arrange the following compounds in order of increaing basicity. (a). CH_(3)NH_(2),CH_(3)NH_(3)^(o+),CH_(3)NH^(-) (b) CH_(3)O^(-),CH_(3)NH^(-),CH_(3)CH_(2)^(-). by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Arrange the following in decreasing order of the basicity.
CH2=CHCH2NH2,CH3CH2CH2NH2,CH≡CCH2NH2AI>II>IIIBII>I>IIICIII>II>IDII>III>IArrange the following in the increasing order of their basic strength CH3NH2,(CH3)2NH,(CH3)3N,NH3
ANH3<(CH3)3N<(CH3)2NH<CH3NH2BNH3<(CH3)3N<CH3NH2<(CH3)2NHCNH3<(CH3)3N<CH3NH2<(CH3)2NHDCH3NH2<(CH3)2NH<(CH3)3N<NH3
Arrange the following in decreasing order of solubility in water:
(CH3)3N,(CH3)2NH,CH3NH2
View SolutionArraange the following compounds in increasing order of dipole moment?
CH3CH2CH3.CH3CH2NH2,CH3CH2OH
View SolutionArrange following in increasing order of basic strength in gas phase
NH3,(CH3)2NH,(CH3)3N,CH3NH2
View SolutionArrange the following compounds in the increasing order of their basic strength. CH3NH−Na+, C2H5NH2, (iso−C3H7)3N and CH3CONH2
View SolutionArrange the following in order of their pKb value
(a)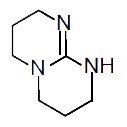 (b) CH3−NH−CH3 (c) H2N−CH=NH
(b) CH3−NH−CH3 (c) H2N−CH=NH
View SolutionArrange the following compounds in the increasing order of their basic strength.
CH3NIFNa+,C2H5NH2,(ISOC3H2)3 and CH3CONH2
View Solution
