What is the mas of sodium bromate and the solution necessary to prepare 85.5 mL of 0.672 N solution when the half cell reaction is BrO−3+56H++6e−→Br−+3H2O.
The correct Answer is:1.446g,0.112M
Topper's Solved these Questions
- OXIDATION & REDUCTION Book:MOTIONChapter:OXIDATION & REDUCTION Exercise:EXERCISE -4(LEVEL-I)
Explore 14 Videos
- OXIDATION & REDUCTION Book:MOTIONChapter:OXIDATION & REDUCTION Exercise:EXERCISE -4(LEVEL-II)
Explore 10 Videos
- OXIDATION & REDUCTION Book:MOTIONChapter:OXIDATION & REDUCTION Exercise:EXERCISE-2 ( LEVEL-II)
Explore 50 Videos
- NOMENCLATURE OF ORGANIC COMPOUNDSBook:MOTIONChapter:NOMENCLATURE OF ORGANIC COMPOUNDSExercise:PREVIOUS YEAR
Explore 8 Videos
- P-BLOCK ELEMENTSBook:MOTIONChapter:P-BLOCK ELEMENTSExercise:Exercise - 4 | Level-I Previous Year | JEE Main
Explore 37 Videos
Similar Questions
How many grams of H2O2 are required to convert 0.1 moles PbS to 0.1 moles PbSO4-
View SolutionConsider a titration of potassium dichromate solution with acidified Mohr's salt solution using diphenylamine as indicator. The number of moles of Mohr's salt required per mole of dichromate is:
View SolutionWhich of the following contains equal number of atoms ?
View SolutionThe IUPAC name of the compound shown below
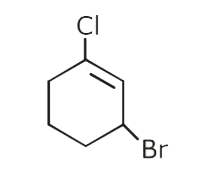
View SolutionThe number of hydrogen atom(s) attached to phosphorus atom in hypophosphorus acid is
View Solution36 " mL of " 0.5 M Br_(2) solution when made alkaline undergoes complete disproportionation into Br^(ɵ) and BrO_(3)^(ɵ) . The resulting solution requried 45 " mL of " As (III) solution to reduce BrO_(3)^(ɵ) to Br^(ɵ) . Given that as (III) is oxidised to As (V), what is the molarity of As (III) solution?
View SolutionFor the chemical reaction:
5Br^(ɵ) + BrO_(3)^(ɵ) + 6H^(o+) rarr 3Br_(2) + 3H_(2)O
Rate =k[Brɵ][BrOɵ3][H⊕]
What is the molecularity and order of reaction with respect to [Brɵ] ?
View SolutionWhat is the weight of sodium bromate and molarity of solution to prepare 85.5 mL of 0.672N solution when half cell reaction are: (i) BrO_(3)^(-)rarr6H^(+)+6e^(-)rarrBr^(-)+3H_(2)O (ii) 2BrO_(3)^(-)+12H^(+)+10e^(-)rarrBr_(2)+6H_(2)O
View SolutionIn the ions equatio, BrO−3+6H++xe−→Br3++3H2O, the value of x is
View Solution(i) What is the mass of sodium bromate and molarity of the solution necessary to prepare 85.4 mL of 0.672 N solution when the half reaction is , BrO_(3)^(-) +6H^(+)+6e^(-) to Br^(-)+3H_(2)O (ii) What would be the mass as well as molartiy if the half cell reaction is, 2BrO_(3)^(-)+12H^(+)+10e^(-) to Br_(2)+6H_(2)O
View SolutionFor the half cell reaction, 2BrO_(3)^(-) + 12H^(+)+10e to Br_(26H_(2)O the equivalent mass of sodium bromate is :
View SolutionThe weight of sodium bromate required to prepare of solution for cell reaction,
View SolutionThe weight of sodium bromate required to prepare 85.5ml of 0.672 N solution for cell reaction , BrO−3+6H++6e→Br−+3H2O, is
View Solution(i) 0.672 N विलयन के 85.5 मिली को बनाने के लिए आवश्यक सोडियम ब्रोमेट का भार तथा विलयन की मोलरता क्या होगी, यदि अर्द्ध-सेल अभिक्रिया निम्न है?
BrO−3+6H++6e−→Br−+3H2O
(ii) यदि अर्द्ध-सेल अभिक्रिया निम्न हो, तो भार तथा मोलरता क्या होगी?
2BrO−3+12H++10e−→Br2+6H2O
View Solution(i) What is the weight of sodium bromate and molarity of solution necessary to prepare 85.5 mL of 0.672 N solution when the half-cell reaction is BrO_(3)^(-) + 6H^(+) + 6e to Br^(-) + 3H_(2) O ? (ii) What would be the weight as well as molarity if the cell reaction is 2BrO_(3)^(-) + 12H^(+) + 10e to Br_(2) + 6H_(2) O ?
View Solution

