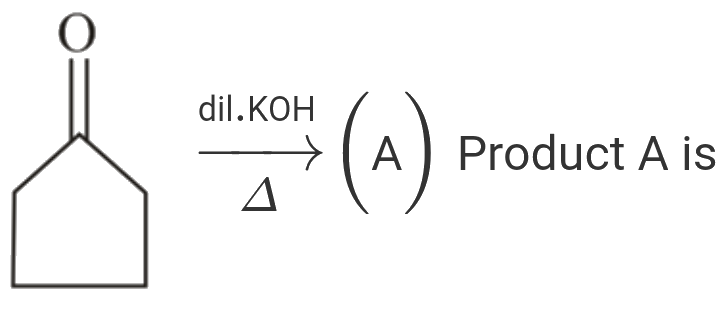Benzoic acid Δ−−−−−−−→NaOH+CaO Product will be




The correct Answer is:A
To determine the product formed when benzoic acid is heated with sodium hydroxide (NaOH) and calcium oxide (CaO), we can follow these steps:
1. Identify the Reactants: The reactants in this reaction are benzoic acid (C6H5COOH), sodium hydroxide (NaOH), and calcium oxide (CaO).
2. Understand the Role of Sodium Hydroxide: Sodium hydroxide is a strong base. When benzoic acid reacts with NaOH, it will deprotonate the carboxylic acid group (-COOH) of benzoic acid. This results in the formation of the benzoate ion (C6H5COO⁻) and water (H2O).
C6H5COOH+NaOH→C6H5COO−+Na++H2O
3. Heating the Reaction Mixture: Upon heating the benzoate ion, the negative charge on the oxygen can undergo resonance. This resonance can lead to the breaking of the bond between the carbon of the carboxylate group and the benzene ring.
4. Decarboxylation: The resonance stabilization allows for the loss of carbon dioxide (CO2) gas. The reaction can be summarized as follows:
C6H5COO−Δ−→C6H6+CO2
Here, the benzoate ion loses CO2 and forms benzene (C6H6).
5. Final Product: The final product of the reaction is benzene.
Conclusion:
Thus, when benzoic acid is heated with sodium hydroxide and calcium oxide, the product formed is benzene (C6H6) along with the release of carbon dioxide gas.
---
Thus, when benzoic acid is heated with sodium hydroxide and calcium oxide, the product formed is benzene (C6H6) along with the release of carbon dioxide gas.
Similar Questions
Knowledge Check
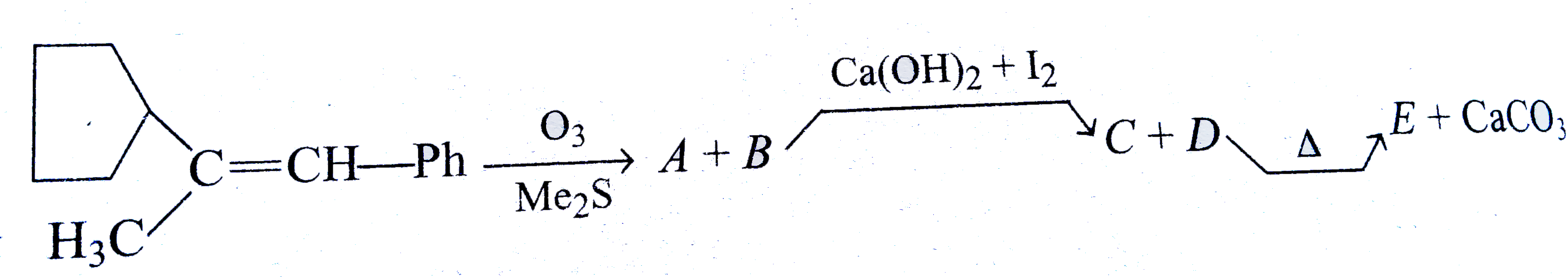
A+BNaOH−−−→Δ Product :A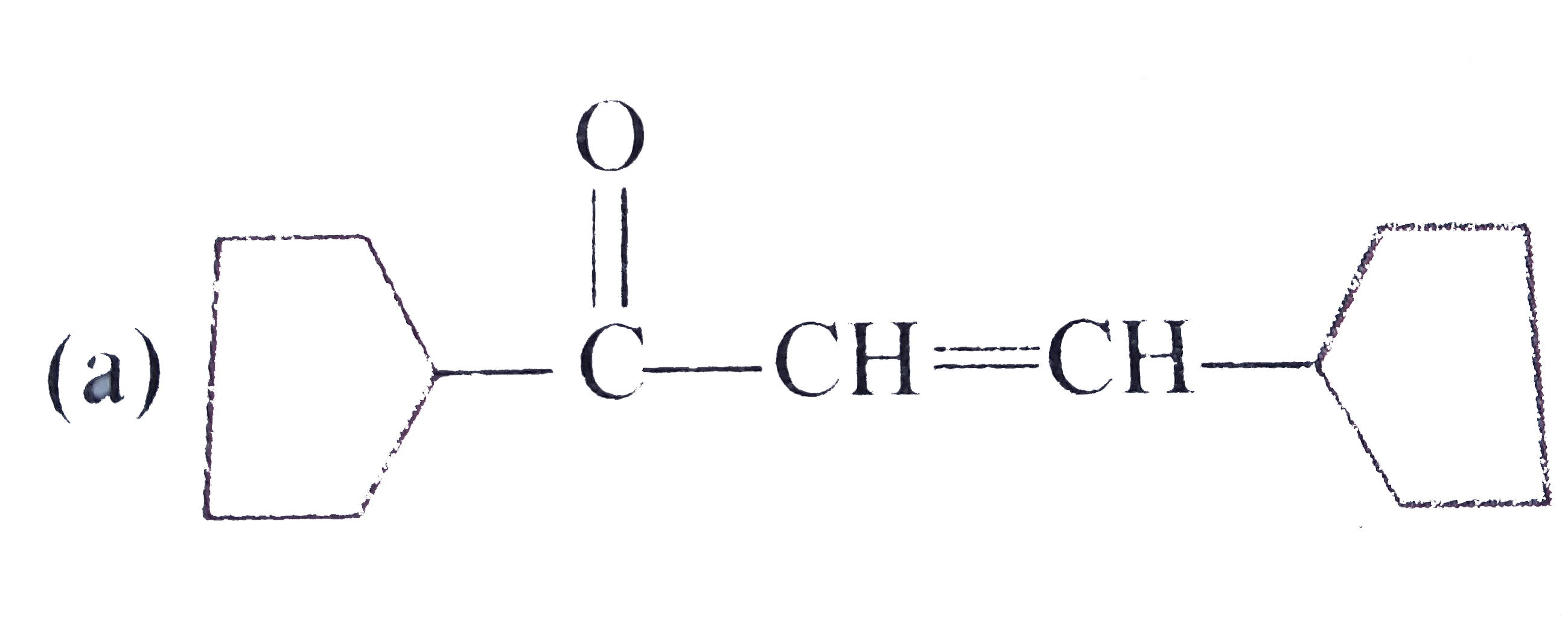 B
B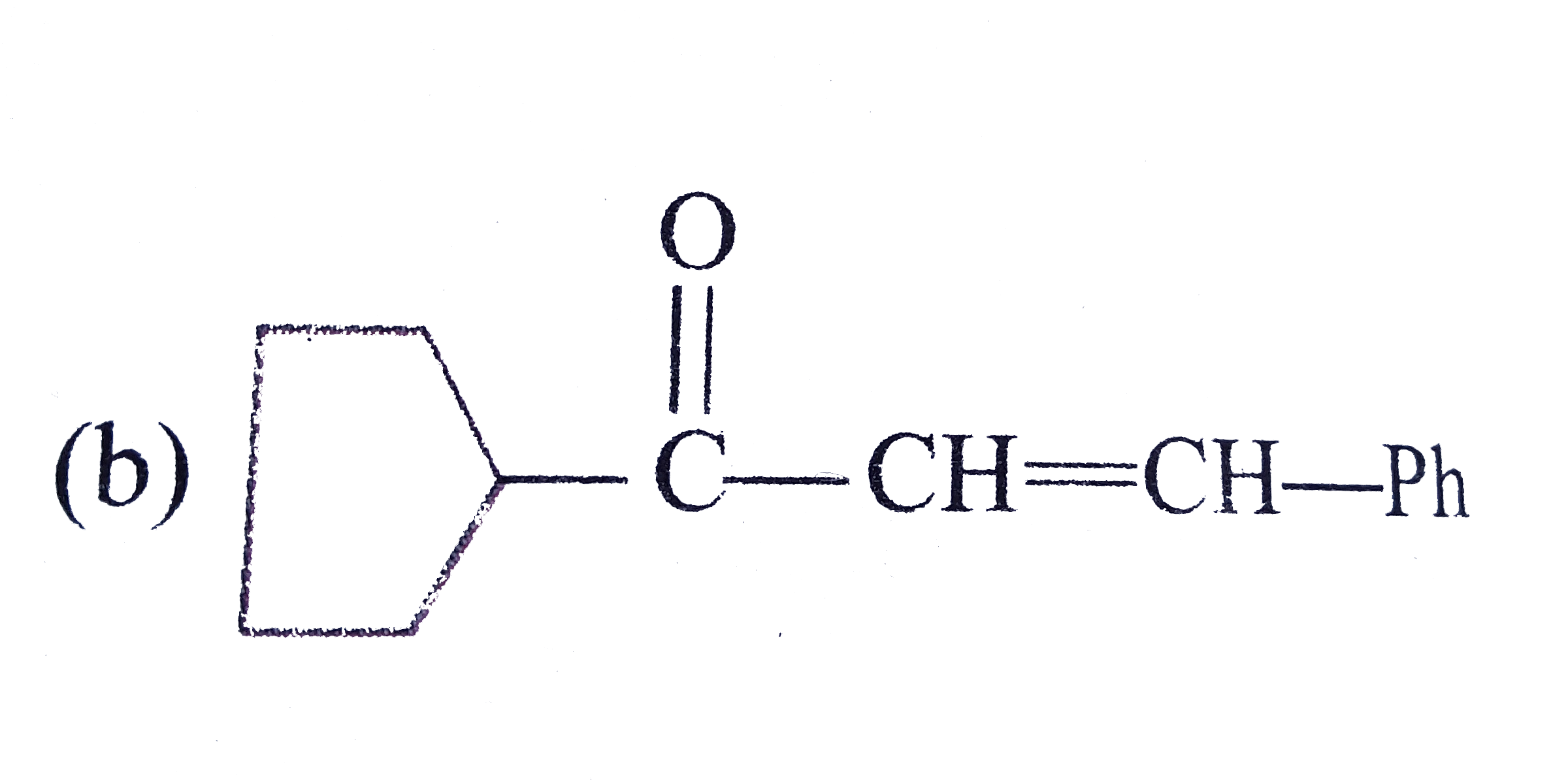 C
C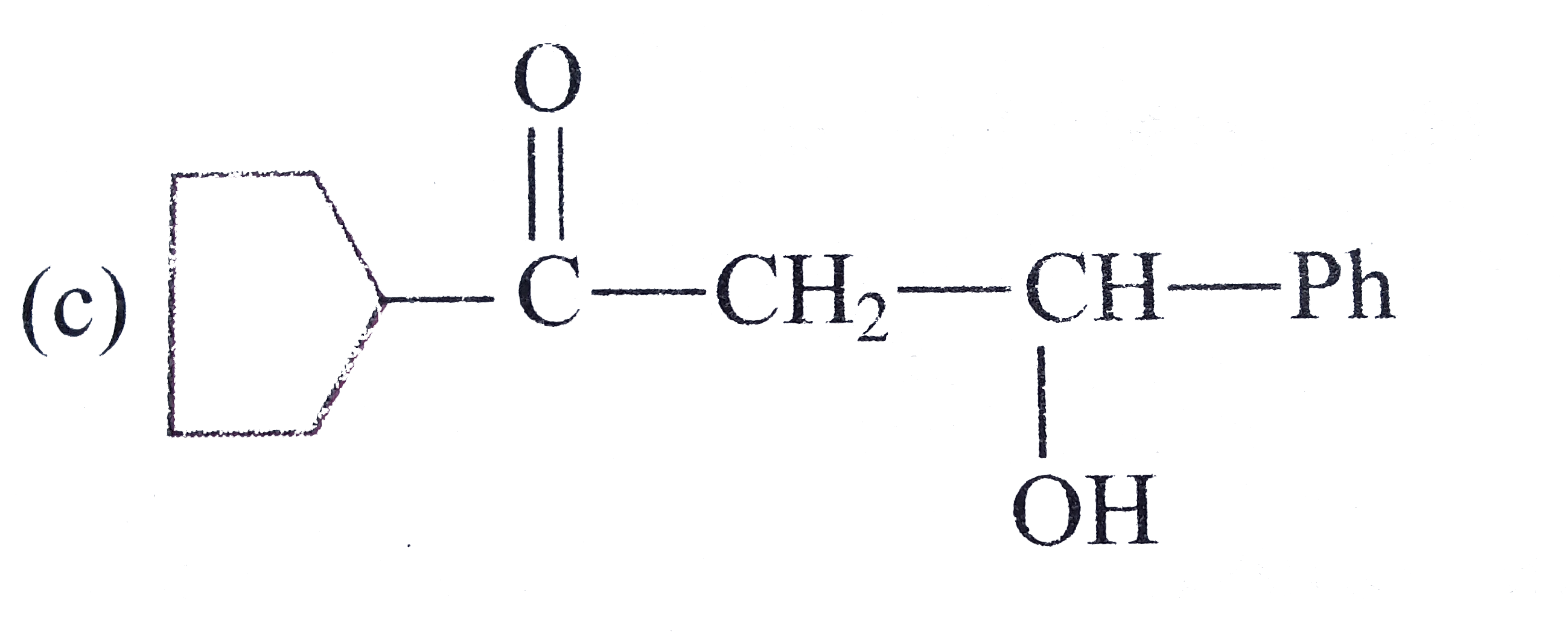 DPh−CH=CH−O∣∣C−Ph
DPh−CH=CH−O∣∣C−PhXHeat−−→P2O5 Malonic acid Δ−−→432KY X and Y in the above reaction sequence are
AC3O2,CH3COOHBCO2,CH3COOHCCO2,CO2DCH3CHO,CH3COOHSalicylic acid Δ−−→ product(s).
Product(s) will be :A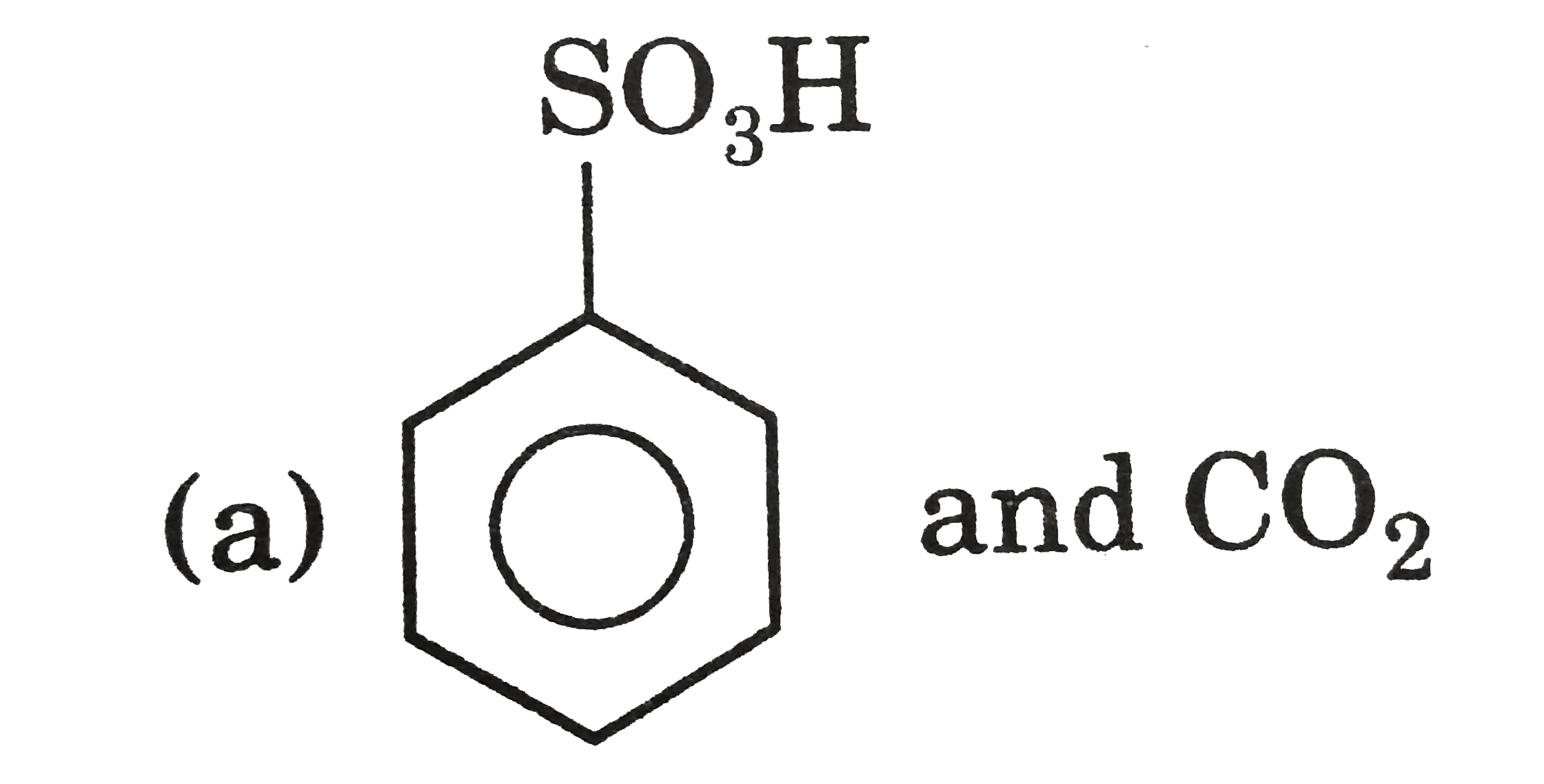 B
B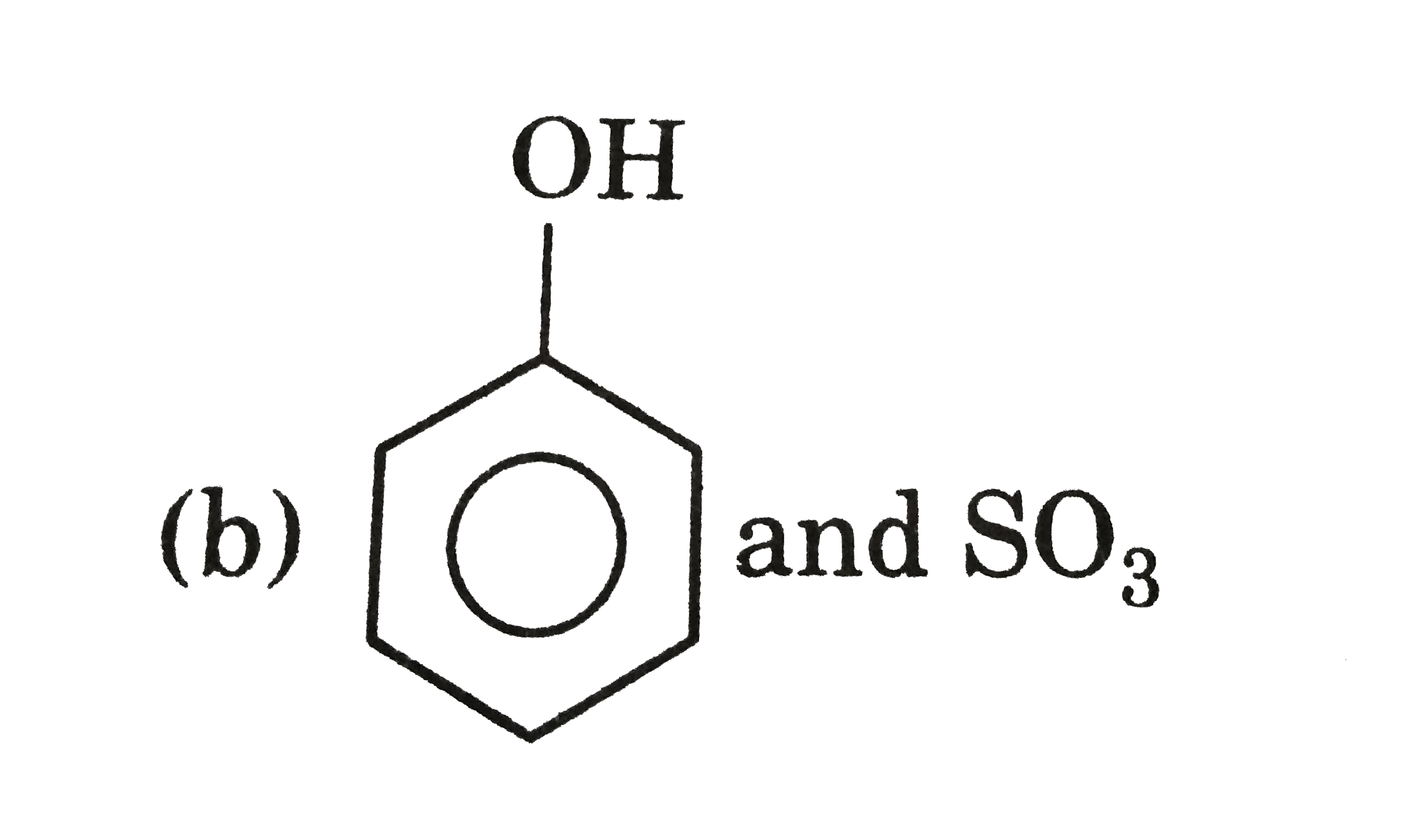 C
C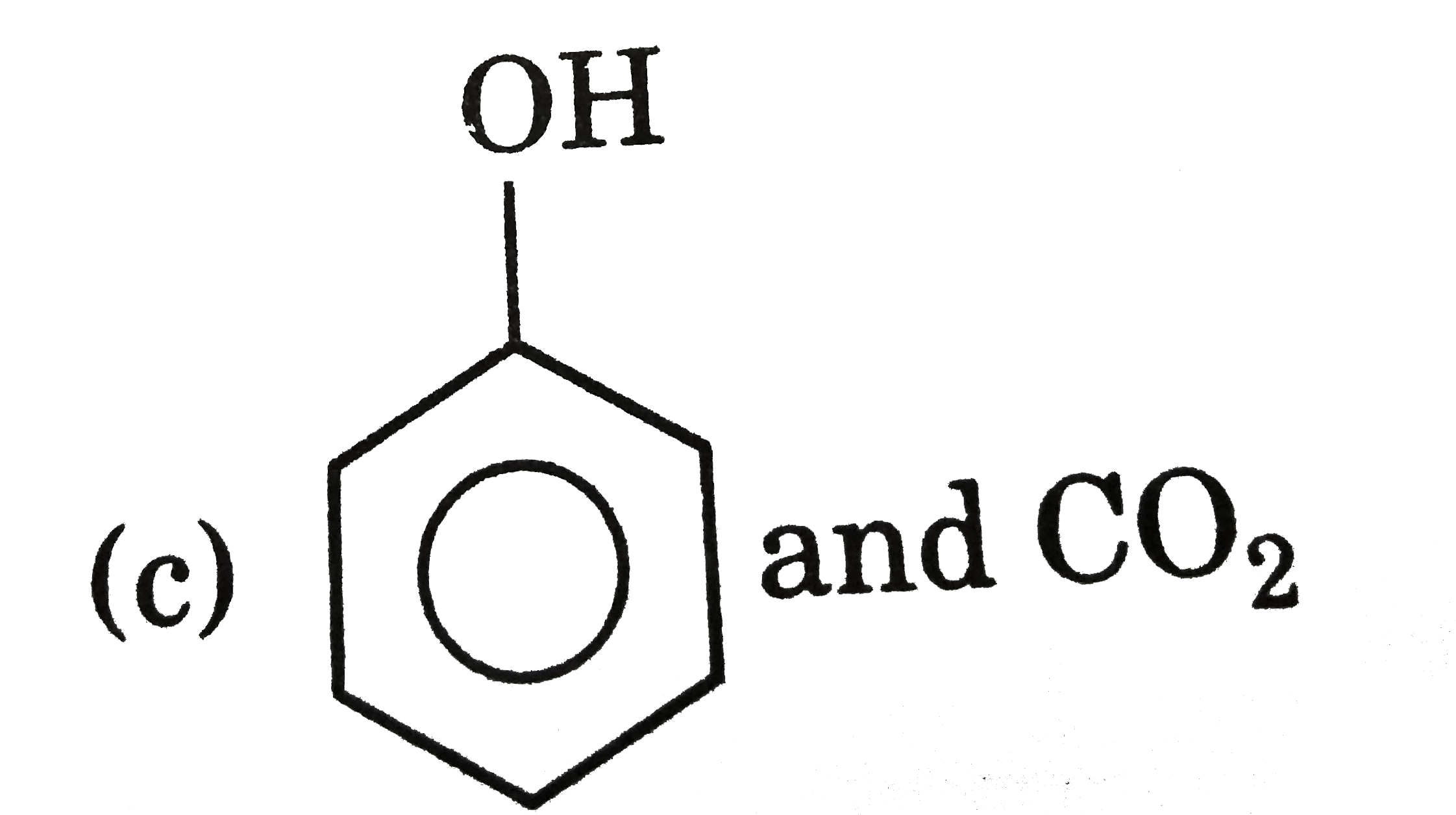 D
D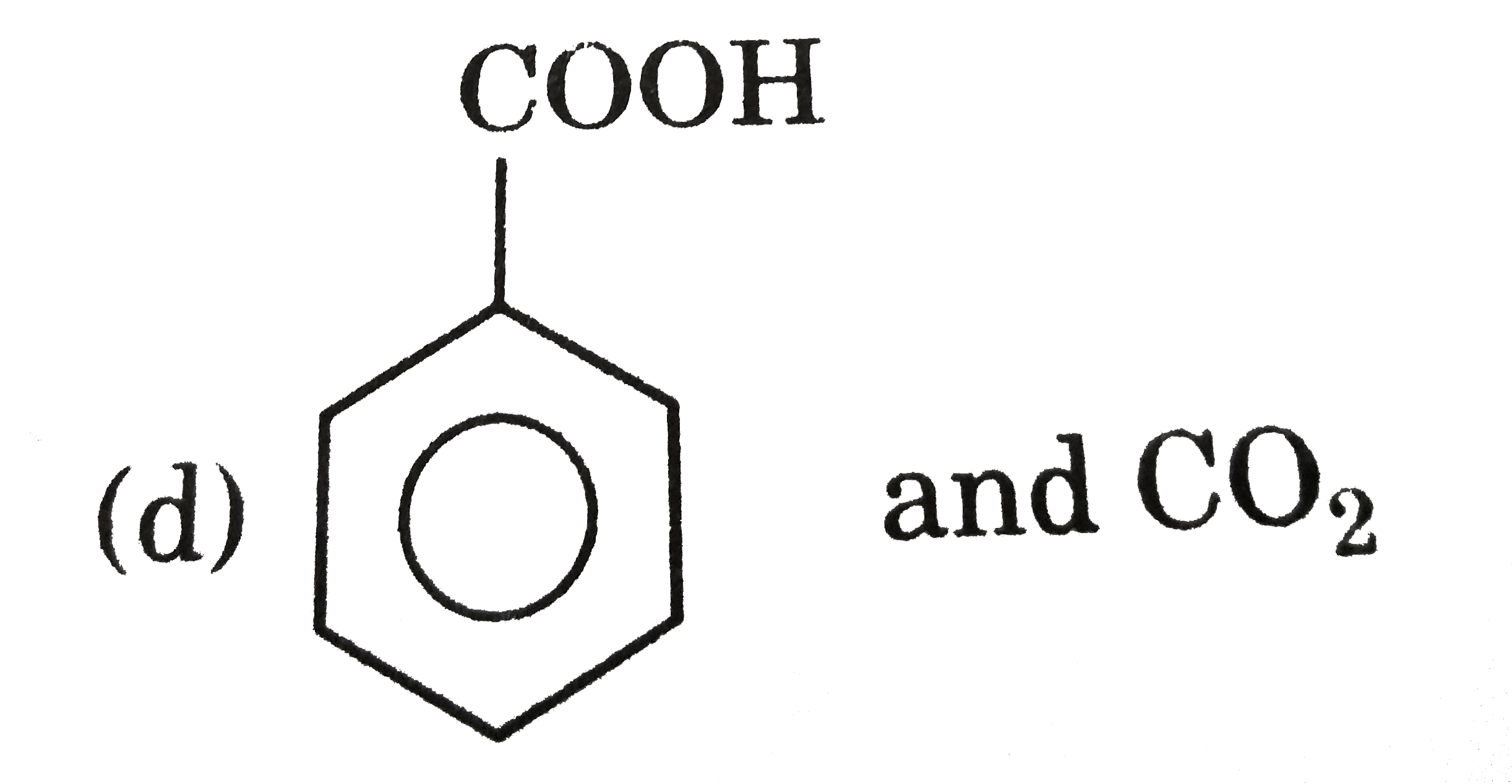
 , AlCl3−−→Δ Product formed as?
, AlCl3−−→Δ Product formed as?
View SolutionIdentify final product in the following reaction,
CH3CH∣OHCOOHΔ−−→ Product
View SolutionPh−CH∣Cl−CH2−Cl(i)Alc.kOH−−−−−−−−−−→(ii)NaNH2(iii)H+ACold alk.KMnO4−−−−−−−−−→BNaOH−−−−−→warmC
The product 'C' eill be
View SolutionCHO∣∣∣COONa+NaOHΔ−−→Products(s)
Identify the products (s) :
View SolutionAccording to the given reaction :
CaCO3(s)10.0gΔ−−→CaO(s)5.6g+CO2(g)xg
What is the value of x ?
View Solution