An ideal gas is transformed from state A(P1,V1) to the state B(P2,V2) through path AB. In this process the work done by the gas is
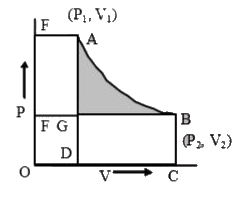
W = area ABCDA
W = area ABEFA
W = area ABGA
W = area ABCOFA
The correct Answer is:A
Similar Questions
A gas with cpcV=γ goes from an intial state (p1,V1,T1) to a final state (p2,V2,T2) through an adiabatic process. The work done by the gas is
View SolutionGraphically shoe the total work done in an expansion when the state of an ideal gas is changed reversibly and isothermally from (pi,Vi) to (pf,Vf). With the help of a pV plot compare the work done in the above case with that carried out against a constant pressure pf.
View Solution
Knowledge Check
A system changes from the state (P1,V1) to (P2,V2) as shown in the figure below. What is the work done by the system –
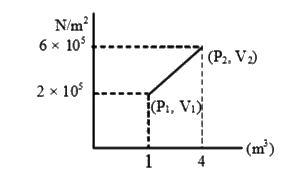 A7.5×105 jouleB7.5×104 ergC12×105 jouleD6×105 joule
A7.5×105 jouleB7.5×104 ergC12×105 jouleD6×105 jouleAn ideal gas expands from state (P1,V1) to state (P2,V2), where P2=2P1 and V2=2V1. The path of the gas is expressed by the following relation,
P=P1[1+(V−V1V1)2]AP1V1B4/3P1V1C2P1V1D4P1V1An ideal gas is taken form the state A(P,V) to the state B(P2,2V) along a straight line path in the P−V diagram.
Select the correct options
(i) The work done by the gas in the process A to B exceeds the work that would be done by it if the system were taken from A to B along the isotherm
(ii) In the T−V diagram, the path AB becomes part of a hyperbola
(iii) In the P−T diagram, the path AB becomes part of a hyperbola
(iv) In going from A to B, the temperature T of the first increases to maximum value and then decreasesA(i),(iii)B(ii),(iii)C(iii),(iv)D(i),(ii),(iv)
An ideal gas is taken from the state A (P, V) to the state B (P/2,2V) along a st. line path as shown in Fig. Select the correct statement from the following:
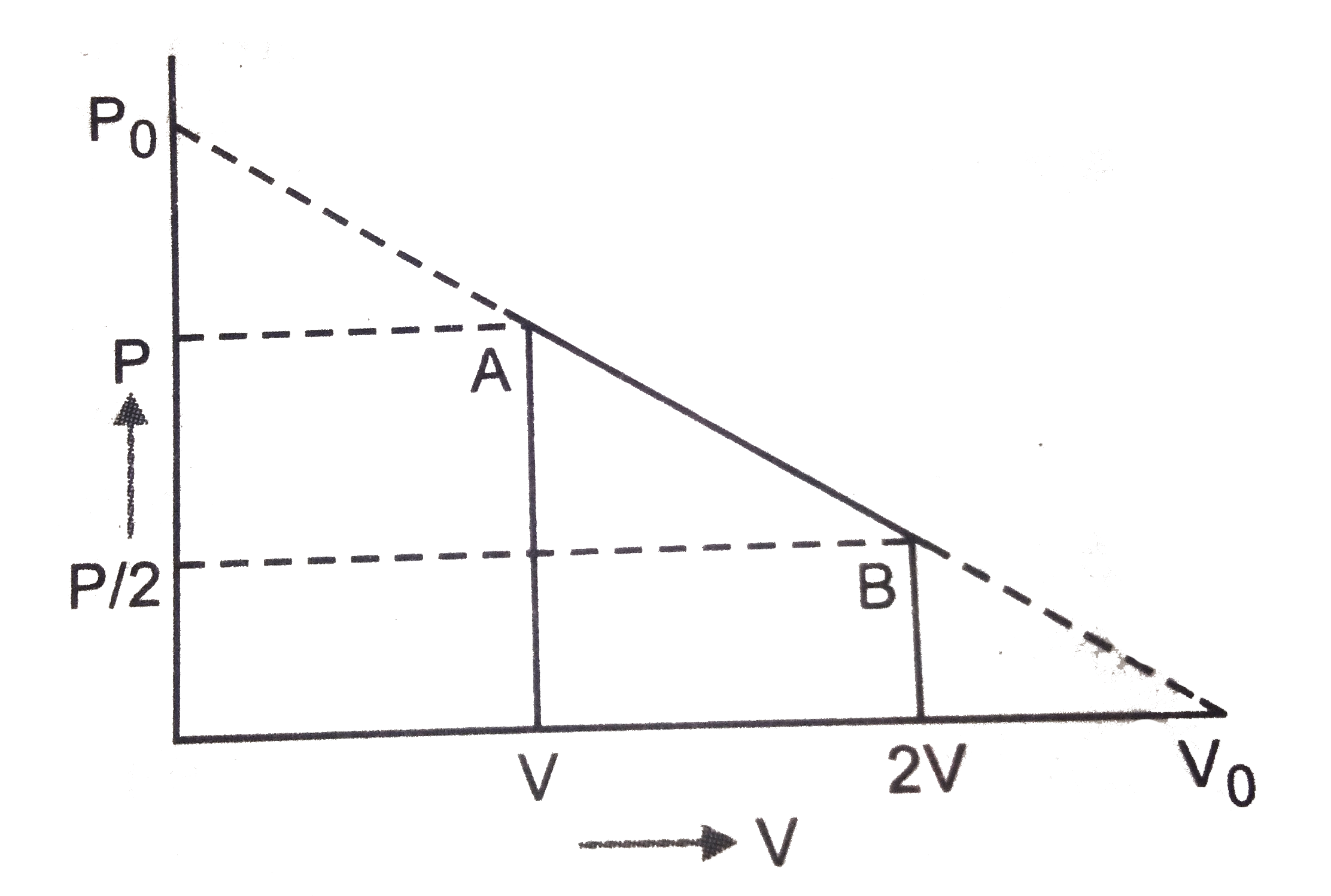
View SolutionAn ideal gas taken from state A(P,V) to the state B(P/2,2V) along a straight path in PV diagram. Select the correct option(s).
View SolutionA system changes from the state (P1,V1) to (P2V2) as shwon in the diagram. The workdone by the system is
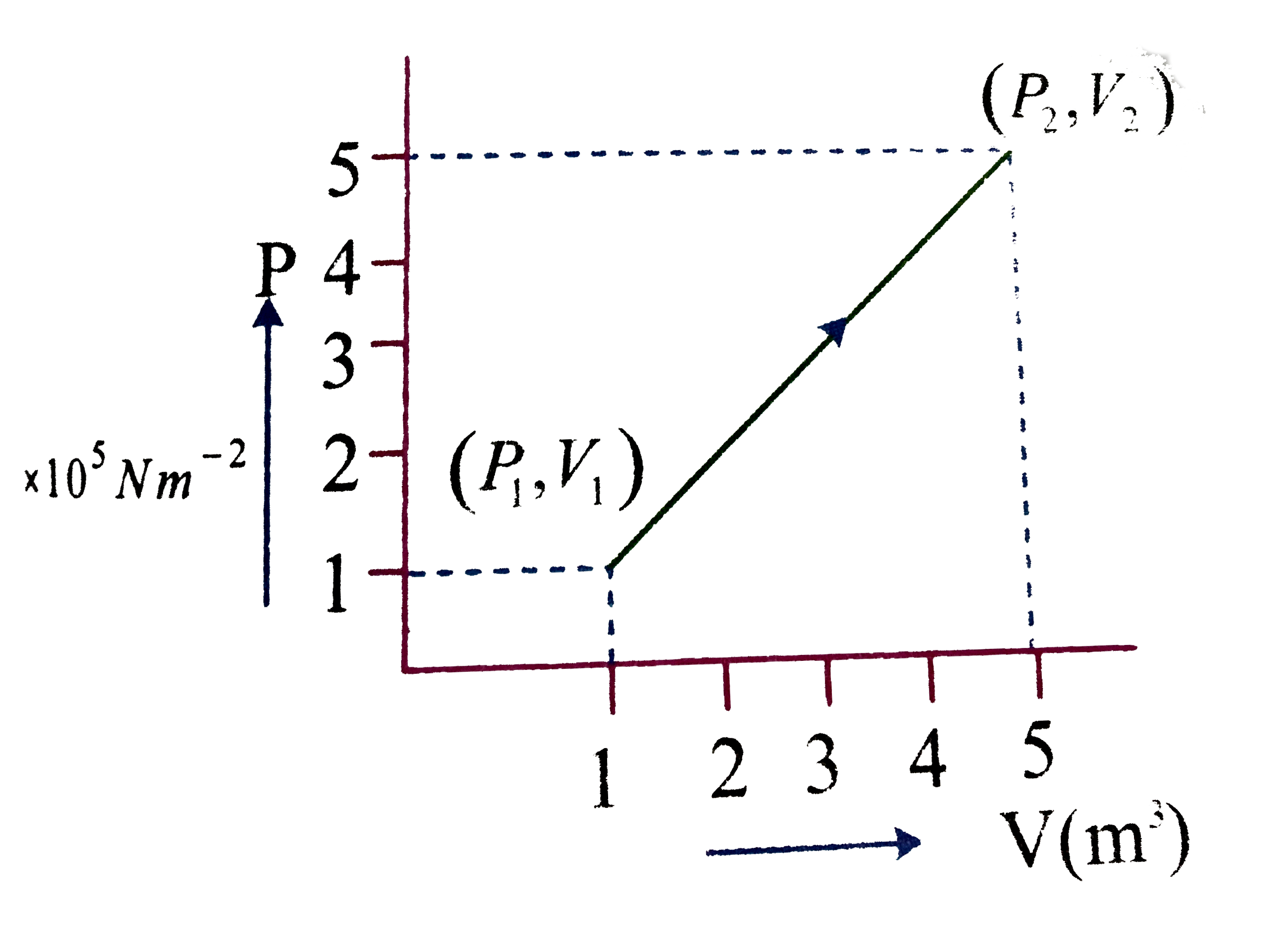
View SolutionOne mole of a gas obeying the expantions P(V-b)=RT is made to expand from a state with (P1,V1) to a state with (P2,V2) along a process that by a straight line on a P-V diagram. Then the work given by:
View Solution2 moles of ideal monatomic gas is carried from a state (P0,V0) to a state (2P0,2V0) along a straight line path in a P- V diagram. The amount of heat absorbed by
View Solution

