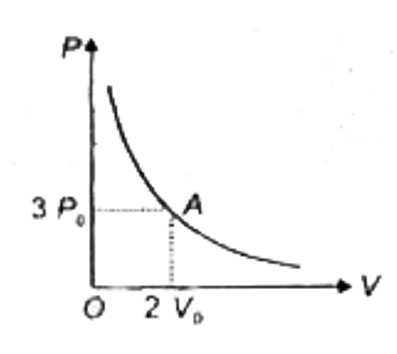
The variation of pressure P with volume V for an ideal monatomic gas during an adiabatic process is hown in figure. At the magnitude of rate of change of pressure with volume is-
A
3P05V0
B
5P03V0
C
3P02V0
D
5P02V0
Text Solution
The correct Answer is:D
|
Similar Questions
Knowledge Check
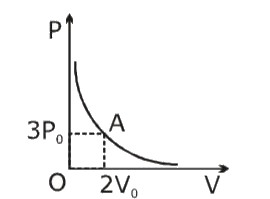
The variation of pressure P with volume V for an ideal diatomic gas is parabolic as shown in the figure. The molar specific heat of the gas during this process is
 A9R5B17R6C3R4D8R5
A9R5B17R6C3R4D8R5The product of pressure and volume of an ideal gas is
Aa constant independent of temperatureBdirectly prouortional to square of its temperatureCdirectly proportional to its temperatureDinversely proportional to its temperatureThe product of the pressure and volume of an ideal gas is
Aa constantBapproximately equal to the universal gas constantCdirectly proportional to its temperatureDinversely proportional to its temperature
An ideal monatomic gas undergoes a cyclic process ABCA as shown in the figure. Graph of pressure versus volume of the cyclic process ABCA is
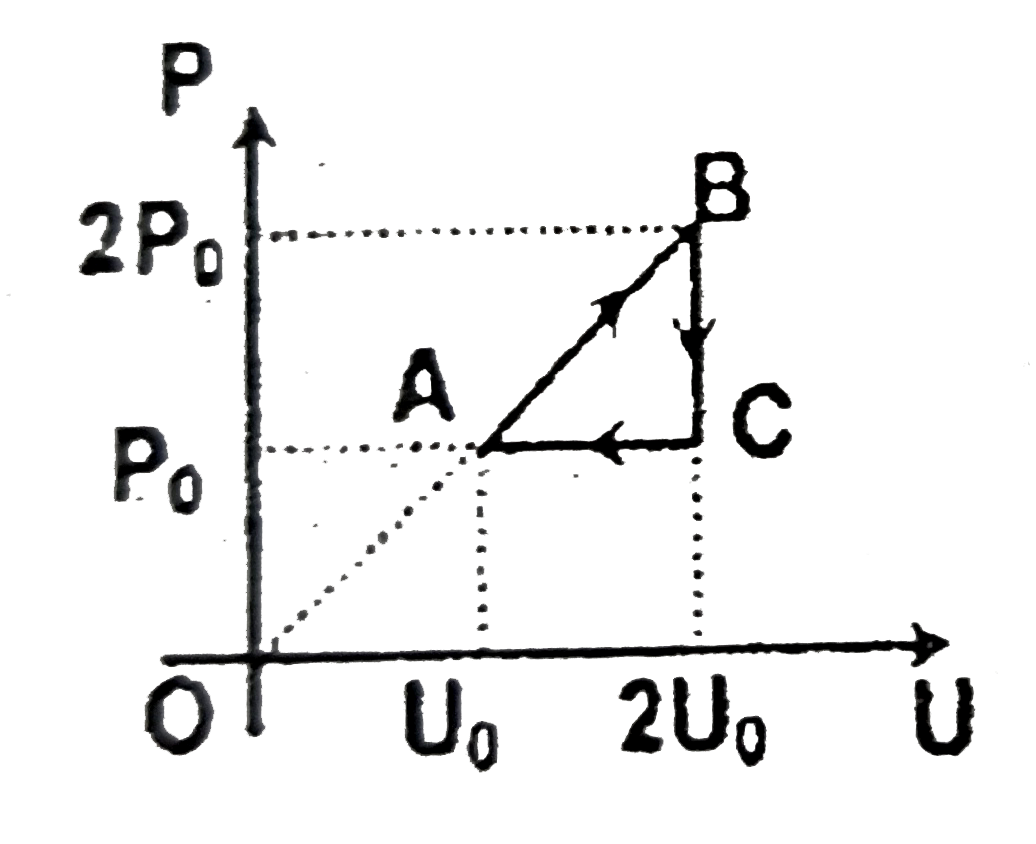
View SolutionThe product of the pressure and volume of an ideal gas is
View SolutionThe relation between the pressure and volume of a gas in an adiabatic process is give by -
View SolutionThe pressure p and volume V of an ideal gas both increase in a process.
View SolutionThe pressure P and volume V of an ideal gas both decreases in a process.
View Solution