Two moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram
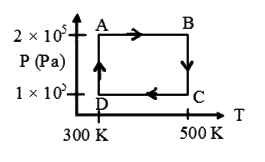
The net work done on the gas in the cycle ABCDA is
zero
276 R
1076 R
1904 R
The correct Answer is:B
Similar Questions
Two moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram
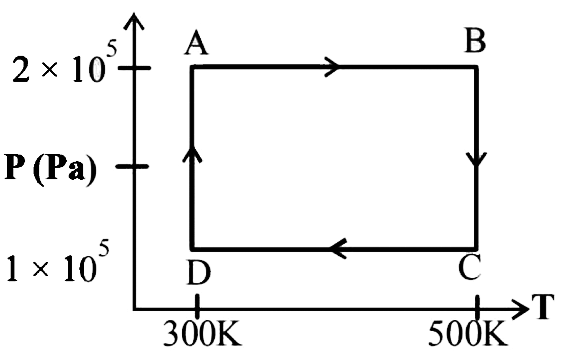
The work done on the gas in taking it from D to A is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram

The work done on the gas in taking it from D to A is :
View Solution
Knowledge Check
Two moles of helium gas are taken over the cycle ABCDA , as shown in the P-Tdigram

The net work done on the gas in the cycle ABCD A is -.AZeroB−276RC1076RD1904RTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram

The work done on the gas in taking it from D to A is :A+414RB−690RC+690RD−414RTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram
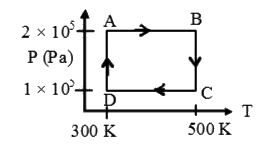
The work done on the gas in taking it from D to A is–A– 414 RB+ 414 RC– 690 RD+ 690 R
Two moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram
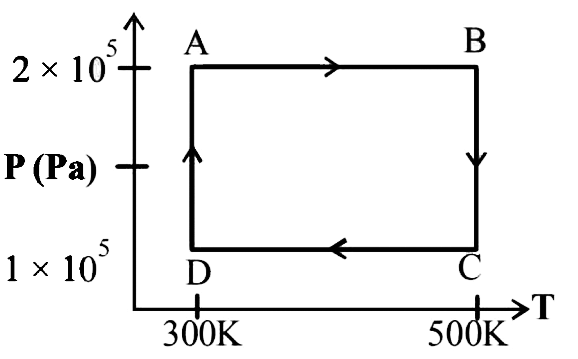
Assuming the gas to be ideal the work done on the gas in taking it form A to B is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram

Assuming the gas to be ideal the work done on the gas in taking it form A to B is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA , as shown in the P-Tdigram

The work done the gas in taking it from D to A is -
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram

Assuming the gas to be ideal the work done on the gas in taking it form A to B is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA , as shown in the P-Tdigram

Assuming the gas to be ideal the work done on the gas in taking if from A to B is -
View Solution

