When a system is taken from state A to state B along the path ACB, 80 k cal of heat flows into the system and 30 k cal of work is done.
If UA=0andUD=40 k cal, find the heat absorbed in the process AD
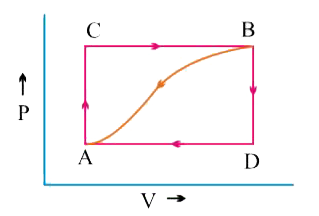
UA=0,UD=40kcal
duAD=40kcal
dwADB=10kcal
dwDB=0sincedV=0
dQAD=40+10=50 k cal
Similar Questions
When a system is taken from state A to state B along path ACB as shown in figure below, 80 J of heat flows into the system and the system does 30 J of work. If E_(D)-E_(A)= -40 J , the heat absorbed in the processes AD and DB are respectively
View SolutionFor a closed system, determine the change in internal energy (DeltaU) when (a) q cal of heat is absorbed by the system and w cal of work is performed on the system (b) q cal of heat is absorbed by the system and w cal of work is performed by the system (c) q cal of heat is evolved by the system and w cal of work is performed on the system (d) heat is not absorbed or evolved by the system, but w cal of work is performed by the system.
View SolutionWhen a system is taken from state A to state B along the path ACB, 80 k cal of heat flows into the system and 30 k cal of work is done.
How much heat flows into the system along path ADB if the work done is 10 k cal ?
View SolutionWhen a system is taken from state A to state B along the path ACB, 80 k cal of heat flows into the system and 30 k cal of work is done. How much heat flows into the system along path ADB if the work done is 10 k cal ? When the system is returned from B to A along the curved path the work done is 20 k cal. Does the system absorb or liberate heat.
View SolutionA system changes its state irreversibly at 300 K in which it absorbs 300 cals of heat. When the same changes is carried out reversibly the amount of heat absorbed is 900 cals. The change in entropy of the system is equal to
View SolutionWhen a system is taken from state I to state f along the path iaf, it is found that Q = 50 cal and W = 20 cal. Along the path ibf, Q = 36 cal. W along the path ibf is

View SolutionWhen the state of a gas adiabatically changed from an equilibrium state A to another equilibrium state B an amount of work done on the stystem is 35 J. If the gas is taken from state A to B via process in which the net heat absorbed by the system is 12 cal, then the net work done by the system is (1 cal = 4.19 J)
View Solution
