Imagine an atom made up of a proton and a hypothetical particle of double the mass of the electron but having the same charge as the electron . Apply the Bohr atom model and consider all possible transitions of this hypothetical particle that will be emitted level . The longest wavelength photon that will be emitted has longest wavelength λ (given in terms of the Rydberg constant R for the hydrogen atom) equal to
95R
365R
185R
4R
The correct Answer is:C
Step by step video, text & image solution for Imagine an atom made up of a proton and a hypothetical particle of double the mass of the electron but having the same charge as the electron . Apply the Bohr atom model and consider all possible transitions of this hypothetical particle that will be emitted level . The longest wavelength photon that will be emitted has longest wavelength lambda (given in terms of the Rydberg constant R for the hydrogen atom) equal to by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
A hydrogen atom moving at speed v collides with another hydrogen atom kept at rest .Find the minimum value of v for which one of the atoms may get ionized, the mass of a hydrogen atom =1.67×10−27kg
View SolutionAs par Bohr model, the minimum energy (in eV) required to remove an electron from the ground state of doubly ionized Li atom (Z=3) is
View SolutionImagine an atom made up of a proton and a bypothetical particle of double the mass of the electron but having the same charge as the electron . Apply the Bohr atom model and consider all possible transitions of this bypothrtical particle that will be emited level . The longest wavwlength photon that will be emidet has longest wavwlength lambda (given in terms of the bytherg constant R for the hydrogen aton) equal to
View SolutionImagine an atom made up of a proton and a hypotnerical particle of double the mass of the electron but having the same charge as the electron. Apply the Bohr atom model and consider all possible transitions of this hypotnetical photon that will be emitted has wavelength lambda (given in terms of the Rydberg constant R for the hydrogen atom) equal to
View SolutionImagine an atom made of a proton and a hypothetical particle of double the mass of the electron but having the same change as the electron. Apply the Bohr atom model and consider all possible transitions of this hypothetical particle of the first excited level. the longest wavelength photon that will be emitted has wavelength [given in terms of the Rydberg constant R for the hydrogen atom] equal to
View SolutionConsider an atom made up of a protons and a hypothetical particle of triple the mass of electron but having same charge as electron. Apply bohr model and consider all possible transition of this hypothetical from second excited state to lower states.The possible wavelengths emitted is (are) (given in term of the Rydberg constant R fr the hydrogen atom)
View SolutionConsider the electron energy level diagram of H-atom Photons associated with shortest and longest wavelength would be emitted from the atom by the transitions labeled
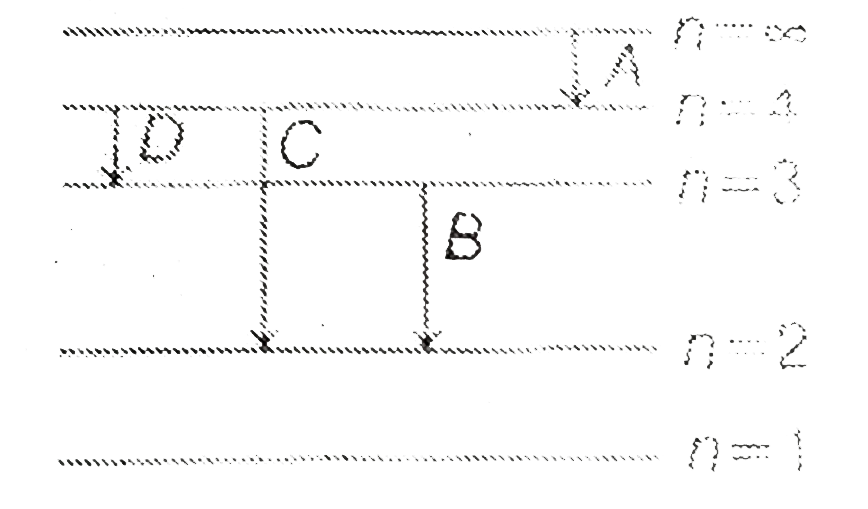
View Solutionएक परमाणु की कल्पना करें जिसमें एक प्रोटॉन है और एक कल्पित कण है जिसके द्रव्यमान , इलेक्ट्रॉन के द्रव्यमान से दुगना है परन्तु आवेश , इलेक्ट्रॉन के आवेश जितना ही है। बोर के परमाणु मॉडल का प्रयोग करें और इस कल्पनिक कण के उत्तेजित स्तर तक सभी संभव संक्रमणों पर विचार करें। सबसे लम्बे तरंगदैर्घ्य का जो फ़ोटॉन उत्सर्जित होगा , उसका तरंगदैर्घ्य λ ( जो इस हाइड्रोजन परमाणु के लिए रिडबर्ग स्थिरांक R के पदों में दिया गया है) है
View SolutionImagine an atom made of protons and a hypothetical particle, in which the mass of the envisaged particle is twice the mass of the electron, but the charge is the same as the charge of the electron. Apply Bohr's atomic model, and consider all possible transitions of the particle to the first excited orbit. The wavelength of the emitted photon of the longest wavelength lambda Is the value of (in terms of the hydrogen R atomic constant R).
View Solutionएक अणु की कल्पना कीजिए जो कि प्रोटॉनों तथा एक काल्पनिक कण से मिलाकर बना है। इस काल्पनिक कण पर इलेक्ट्रॉन के बराबर आवेश है। तथा द्रव्यमान इलेक्ट्रॉन का दोगुना है। बोहर के परमाणु मॉडल का प्रयोग करते हुए इस कण के प्रथम उत्तेजन स्तर पर आने वाले सभी सम्भव संक्रमणों पर विचार कीजिए। सबसे लम्बी तरंगदैर्ध्य का फोटॉन जो कि उत्सर्जित होगा उसकी तरंगदैर्ध्य 1 रिडबर्ग नियतांक R(हाइड्रोजन परमाणु के लिए) के पदों में होगी।
View SolutionImagine an atom made up of a proton and a hypothetical particle of double the mass of the electron but having the same charge as the electron . Apply the Bohr atom model and consider all possible transitions of this hypothetical particle that will be emitted level . The longest wavelength photon that will be emitted has longest wavelength lambda (given in terms of the Rydberg constant R for the hydrogen atom) equal to
View SolutionConsider the electron energy level diagram of H-atom Photons associated with shortes and longest wavelength would be emitted from the atom by the transitions labeled
View SolutionConsider an atom made up of a protons and a hypothetical particle of triple the mass of electron but having same charge as electron. Apply bohr model and consider all possible transition of this hypothetical from second excited state to lower states.The possible wavelengths emitted is (are) (given in term of the Rydberg constant R for the hydrogen atom)
View SolutionImagine an atom made of a proton and a hypothetical particle of double the mass of the electron but having the same change as the electron. Apply the Bohr atom model and consider all possible transitions of this hypothetical particle of the first excited level. the longest wavelength photon that will be emitted has wavelength [given in terms of the Rydberg constant R for the hydrogen atom] equal to
View SolutionImagine an atom made up of a proton and a hypothetical particle of double the mass of the electron but having the same charge as the electron. Apply the Bohr atom model and consider all possible transitions of this hypothetical particle to the first excited level. The longest wavelength photon that will be emitted has wavelength λ (given in terms of Rydberg constant R for the hydrogen atom) equal to
View SolutionImagine an atom made up of a proton and a hypothetical particle of double the mass of the electron but having the same charge as the electron. Apply the Bohr atom model and consider all possible transitions of this hypothetical particle to the first excited level. The longest wavelength photon that will be emitted has wavelength lamda (given in terms of the Rydberg constant R for the hydrogen atom) equal to .
View Solution

